Раздел A ниже содержит (а) документы, распечатанные судьей Пегги Куо в январе 2021 года в ответ на ходатайство Reuters America LLC о вскрытии судебного разбирательства по делу Пропеции в США, (b) документы по тому же судебному процессу, добровольно опубликованные ответчиками для общественности в октябре 2020 года. Merck & Co., Inc. и Merck Sharp & Dohme Corp., и (c) несколько сопутствующих документов, в которых отмечаются важные события по делу. Раздел B содержит сообщения средств массовой информации, которые отслеживают эту историю на протяжении трех десятилетий, пока она распространилась за пределы Соединенных Штатов в Канаду, Францию, Германию и Израиль.
«Согласно Плану по управлению рисками компании Merck, с 1998 по 2008 год около 4,6 миллиона мужчин получали финастерид в дозе 1 мг. Согласно общедоступной информационной панели системы отчетности FDA о побочных эффектах, по состоянию на июнь 2021 года было 10 295 серьезных побочных эффектов финастерида, Майкл С. Ирвиг, доктор медицинских наук, профессор Гарвардской медицинской школы и эндокринолог в Медицинском центре Бет Исраэль Дьяконис, написал в обзоре «Андрология», озаглавленном «Как стандартный фармаконадзор не смог выявить стойкие сексуальные побочные эффекты финастерида».
Столкнувшись с данными о том, что их продукт может вызвать стойкую эректильную дисфункцию, в 2009 году отдел безопасности по управлению рисками в Merck получил возможность предпринять дальнейшие действия для решения проблемы. Понимая, что во многих отчетах о побочных эффектах отсутствует адекватная информация, команда могла бы рекомендовать проведение дополнительных тщательных проспективных исследований финастерида для получения необходимой информации … Вместо того, чтобы искать правду о серьезном и жизненно важном побочном эффекте, спланированные действия [о]тдела были стандартным фармаконадзором еще два года. Это решение трудно оправдать, поскольку стандартный фармаконадзор не смог предоставить адекватную информацию о сотнях случаев с 1998 по 2008 год. FDA также несет определенную ответственность, поскольку оно не советовало или не требовало от Merck проведения дополнительных исследований по безопасности».
Фонд ПФС предоставил эти документы в качестве ресурса для заинтересованных сторон, включая пациентов без ПФС и их близких, поставщиков медицинских услуг, научных исследователей, должностных лиц федеральных регулирующих органов и представителей средств массовой информации.

A. Документальное подтверждение
B. СМИ гонятся за правдой
| The New York Times (US) December 23, 1997 A Pill for Male Pattern Baldness Wins Approval From the FDA |  | The Food and Drug Administration announced today that it had given Merck & Company permission to sell a tiny tan octagonal tablet that, experiments show, either promoted the growth of hair or at least stopped hair loss in 83 percent of men. Critics…complain that no long-term studies have been conducted on the drug. |
| LawersAndSettlements.com (US) February 12, 2011 Propecia and Proscar Sexual Dysfunction Class Action Filed |  | A class lawsuit has been [filed] in the Supreme Court of British Columbia, Canada, by Vancouver resident Michael Miller against Merck Frosst Canada and its affiliated companies…on behalf of Canadian men who used Propecia or Proscar and suffered continuing sexual dysfunction. Miller, who is in his early 20s, [says] ‘My sexual functioning has not recovered, I have seen specialists and have tried treatments but nothing has worked.’ |
| Hartford Courant (US) March 3, 2011 Lawsuit Blames Hair Loss Drug For Sexual Dysfunction And Mental Problems |  | A Connecticut law firm has filed a lawsuit against pharmaceutical manufacturer Merck & Co. on behalf of men who have taken Propecia, a prescription drug that combats hair loss but, its critics say, can also cause severe side effects such as sexual dysfunction and mental impairment. One Connecticut patient…said that he stopped taking Propecia three years ago after experiencing trouble breathing, sexual dysfunction, anxiety attacks and memory loss. He asked not to be named because his mother still does not know of the severe side effects the drug caused. |
| LawyerAndSettlements.com (US) February 24, 2012 Propecia Website Down |  | Patients looking for information about Propecia may have to search more than anticipated now that the Propecia website has been taken down. The move has sparked speculation about Propecia lawsuits and Propecia side effects. Although the warning label in the US alerts patients to the possibility of sexual side effects, the label also states that the side effects are temporary… Warning labels in Europe reportedly contain information that the sexual side effects could be permanent. |
| The Canadian Press (Canada) April 2, 2013 Class Action Over Baldness, Prostate Drug Side Effects Gets the Go-ahead |  | Men who suffered ongoing erectile dysfunction after taking prescription drugs to treat prostate problems and male pattern baldness will be able to pursue a class-action lawsuit against the drug maker, a B.C. judge has ruled. ‘The plaintiff asserts that the defendants were aware of the long-term side effects and that the warnings given in Canada were inadequate,’ the judge noted in his ruling. |
| Toronto Star (Canada) April 19, 2013 Canadian Men in Class Action Lawsuits Say Drug They Took for Baldness Has Left Them Impotent |  | More than 500 Canadian men feel they weren’t warned properly about a prescription baldness medication they say has left them impotent, even years after they stopped taking it. Sean Ramsaran, 26, was prescribed Propecia by his dermatologist in 2009…and now calls himself ‘a useless person…I have nightmares, flashbacks to experiences I had while on the drug.’‘[Merck] acted responsibly and appropriately with respect to Propecia and Proscar throughout the development, marketing and post-marketing monitoring of these medicines,’ wrote spokeswoman Lainie Keller in an email. She added there is no scientific data showing the drugs cause persistent impotence after discontinuing use. |
| Men’s Journal (US) September 2015 Are Hair-Loss Drugs Safe? |  | Since 2011, 1,245 lawsuits have been filed against Propecia’s manufacturer, Merck, alleging that the company failed to warn users of a constellation of sexual and cognitive side effects — which patients and physicians call Post-Finasteride Syndrome… This spring, the National Institutes of Health added PFS to its rare-diseases database. And in March, a California woman filed the first wrongful death suit against Merck. Merck said the company «stands behind the demonstrated safety and efficacy profile of Propecia.» In recent years, it also added depression and persistent sexual problems to its list of possible side effects, deep in the fine print. It intends to defend itself vigorously when the first cases go to court, likely in 2016. The company will undoubtedly argue that millions use Propecia without harm, and that serious problems are rare. Not rare enough, says Steven Rossello, a 32-year-old who filed the first suit against Merck, in 2011. |
| L’Obs (France) November 23, 2017 (Part 1) July 21, 2018 (Part 2) Prescription Hair-loss Medication Propecia Accused of Ruining Lives English translation here: Part 1 Finasteride, the Controversial Drug that Medical Authorities Continue to Defend English translation here: Part 2 |  | In June 2016, 25-year-old Romain took his life. According to his family, his death can be blamed on post-finasteride syndrome. Finasteride is sold by Merck & Co., which may now be subject to civil or criminal prosecution in France. [F]inasteride trials using a control group were at first limited to a duration of one year, which was then extended for another year–but only for certain patients: those who were healthy. So these trials…all had one failing: they included people in good health and avoided the inclusion of other categories of patients.” |
| Die Zeit (Germany) February 22, 2018 A Pill the Doctor Gave Me Destroyed My Life. Patients are now filing lawsuits against the pharmaceutical company English translation here |  | The night Thorben Weber lost his manhood ended with, paradoxically, a stunning sunrise in front of his bedroom window. It was July 2015 when he drew his curtains open and looked out at the gently rolling hills of the Black Forest. His body looked the same as always. But nothing worked like it used to. In the ensuing weeks, Weber lost his girlfriend, his apartment and his self-respect. Neurologists, urologists, dermatologists…they all said Weber was just imagining the discomfort. It took weeks before a professor of andrology at Münster University…made a diagnosis that could explain all the symptoms. ‘You have the Post-Finasteride Syndrome,’ said Michael Zitzmann. |
| SonntagsZeitung (Switzerland) December 22, 2018 Finasteride Was Once Considered a Miracle Cure for Hair Loss. Now It’s Going to Court English translation here | 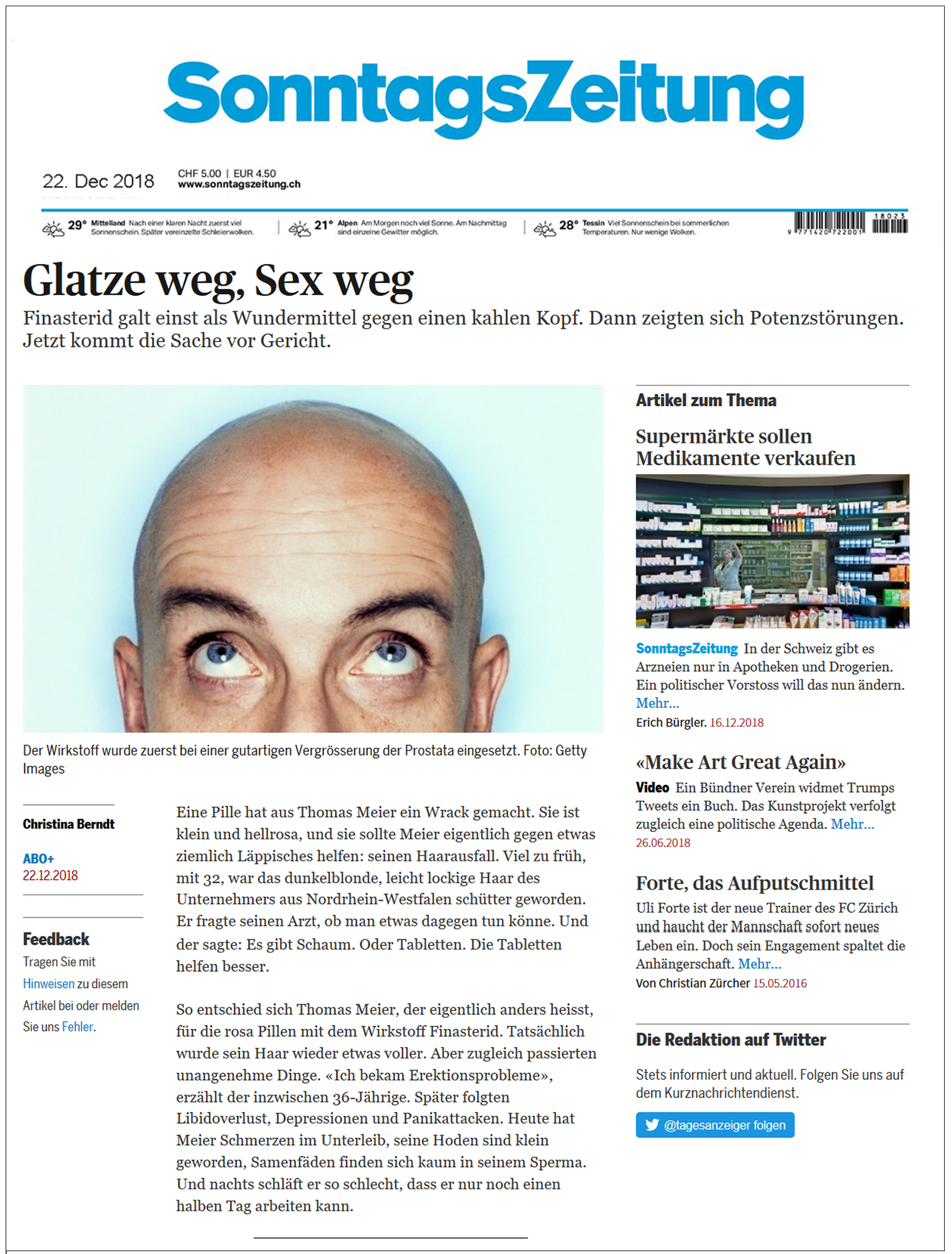 | A pill has destroyed Thomas Meier. It’s small, it’s pink and it was supposed to help him with something silly: hair loss…His hair got a little bit thicker, but unpleasant things started happening. ‘I was having erectile problems’ [he] explained. Later on, this was followed by loss of libido, depression and panic attacks. According to one [study], finasteride users were three times more prone to develop mental health problems than men in the control group… In July 2018 the manufacturers ultimately added to the leaflets that the sexual side effects were nonreversible… And yet the medicine is still on the market. |
| Le Parisien (France) March 3, 2019 Propecia: This Mother Accuses the Hair-loss Treatment of Being Responsible for her Son’s Suicide English translation here |  | This is the story of Romain Mathieu, a young man who for several years took Propecia, a drug… that can lead to serious side effects ranging from sexual dysfunction to depression… Romain Mathieu ended his life in 2016, but his case will soon be brought to justice, along with two others. During a final hormonal assessment, his doctor was bewildered: Roman had testosterone levels equivalent to those of an 80-year-old man. |
| Mediapart (France) March 13, 2019 The Victims of a Hair-loss Treatment Are Suing MSD English translation here | 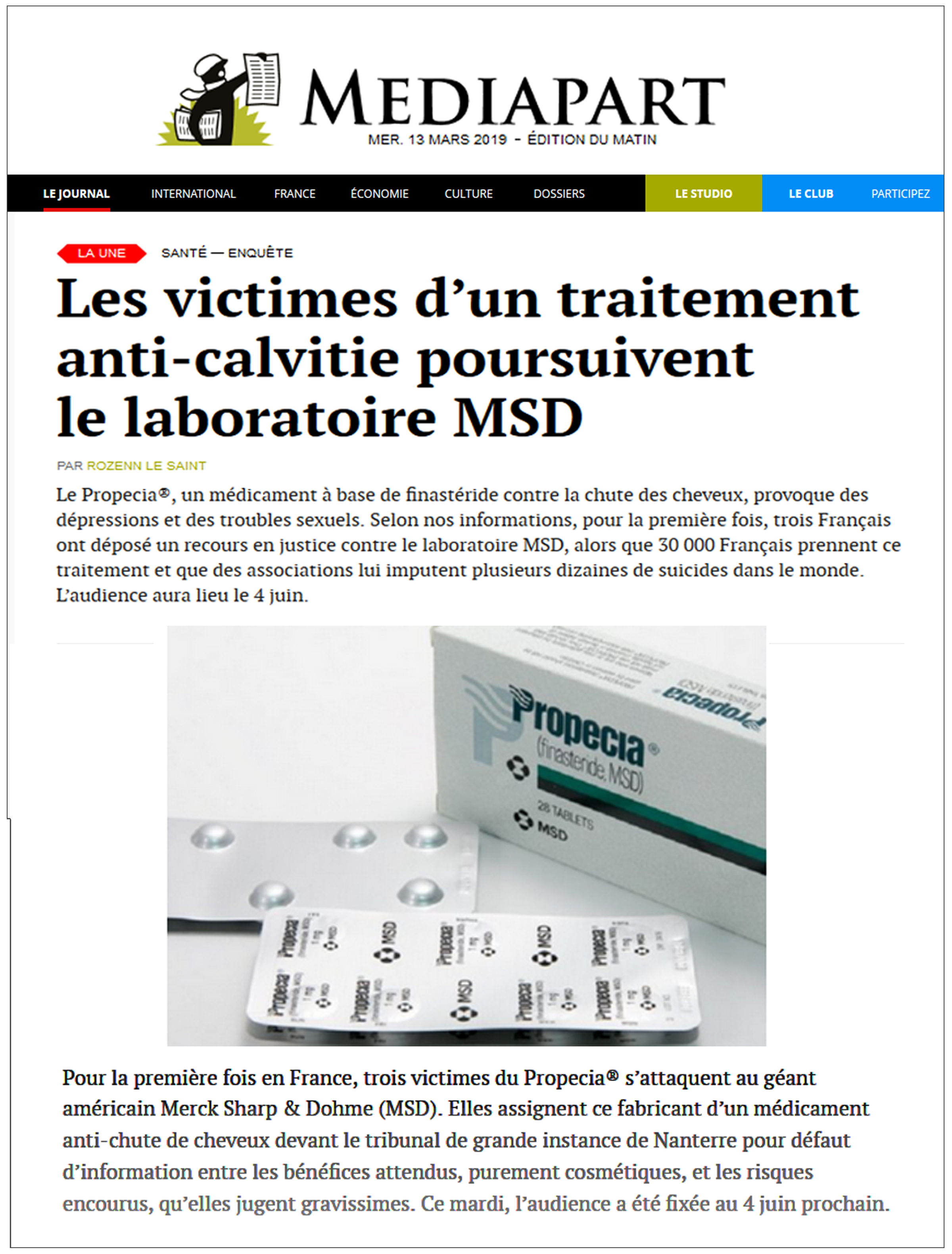 | For the first time in France, three Propecia victims are suing American pharmaceutical giant Merck Sharp & Dohme. They are…claiming a lack of information about its expected benefits (purely cosmetic), and the risks involved when taking it, which they claim are extremely serious. ‘Before he committed suicide…Romain left a note telling me, If you have the courage, fight,’ says his mother, Sylviane Million-Mathieu. |
| Deutsche Presse-Agentur (Germany) May 22, 2019 Hair-loss Trial: Plaintiff Seeks Compensation English translation here | 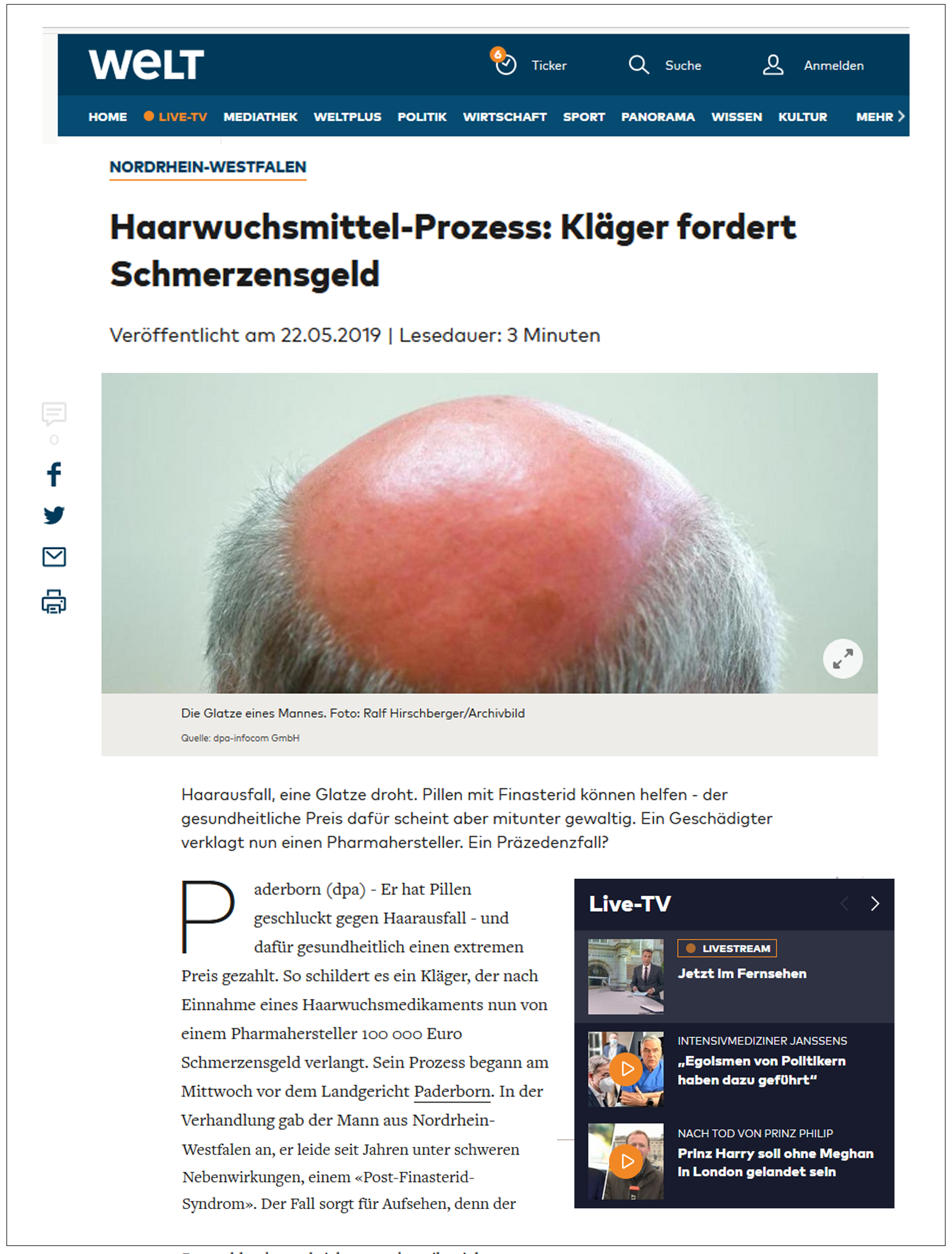 | “I took a hair-loss pill and paid the ultimate price—with my health.” This is how a victim of the hair loss medication describes his situation. Now he is demanding 100,000 euros from the pharmaceutical manufacturer as compensation. On the first day of the trial…the plaintiff was met with partial success: The defending pharmaceutical companies must hand over detailed information on effects, interactions, side effects, and information about all known suspected cases. |
| Reuters (US) September 11, 2019 Court Let Merck Hide Cecrets About a Popular Drug’s Risks |  | On the morning of March 5, 2013, about 45 minutes before his wife got home, John Pfaff stepped onto the railroad tracks a block away and into the path of a southbound Amtrak train. He was killed on impact. Kelly Pfaff blames Merck for her husband’s death at age 40. In a lawsuit filed in 2015, she alleges that the pharmaceuticals company for years knew but concealed from the public that Propecia could cause the persistent sexual dysfunction and depression that led to her husband’s suicide. [C]onfidential documents reviewed by Reuters accuse Merck of exaggerating the drug’s safety record… [They] allege that in revisions to the drug’s original 1997 label, Merck understated the number of men who experienced sexual symptoms in clinical trials, and how long those symptoms lasted. |
| Reuters (US) January 25, 2021 Judge Orders Merck Documents on Anti-baldness Drug Propecia Unsealed |  | A US judge granted a Reuters request to unseal Merck & Co. documents produced in lawsuits related to its anti-baldness treatment Propecia, finding that the public’s right to access outweighed the drug maker’s arguments for keeping the information secret. [P]laintiffs’ lawyers cited internal company communications to allege that in revisions to the drug’s original label, Merck understated the number of men who experienced sexual symptoms in clinical trials and how long those symptoms lasted. Merck settled before responding to the allegations in court. ‘Merck’s arguments for keeping the documents sealed,’ [Judge Peggy] Kuo wrote, ‘are so weak that they would not overcome even a low presumption of access under the common law.’ |
| Reuters (US) February 13, 2021 Exclusive: Merck Anti-baldness Drug Propecia Has Long Trail of Suicide Reports, Records Show |  | Newly unsealed court documents and other records show that Merck & Co and US regulators knew about reports of suicidal behavior in men taking the company’s anti-baldness treatment Propecia when they decided not to warn consumers of those potential risks in a 2011 update of the popular drug’s label. Since…2011…the FDA has received more than 700 reports of suicide and suicidal thoughts among people taking Propecia or generic versions… Those included at least 100 deaths. [I]n the first 14 years the drug was on the market, the agency received 34 such reports, including 10 deaths… To this day, the US label contains no mention of suicide or suicidal thoughts. |
| Daily Mail (UK) May 21, 2021 Man, 24, Who Said He Was Depressed After Taking Hair Loss Tablet Finasteride Over Five Years Killed Himself by Jumping off Bridge, Inquest Hears |  | A young man who claimed to be depressed after taking a hair loss tablet for five years killed himself by jumping off a bridge, an inquest heard. Jack Hogg, 24, from Exeter, Devon, was not losing his hair but had been self medicating with finasteride. In the months before his death he became concerned about its side effects including depression. The inquest heard Jack had researched post-finasteride syndrome online which gave a very bleak outlook and led him to believe he had ruined his life. He died from multiple injuries in the fall from 24 metres from the road bridge. |
| WDR (Germany) June 30, 2021 Tricks of the Beauty Industry |  | Many of those affected have come together in a model class-action lawsuit. This is paid for by a litigation financier from Cologne. There we met attorney Stephan Bensalah. It’s an enormous emotional challenge. You have to imagine: These young men are extremely damaged, some of them are unable to work and their relationships have suffered. Going alone in this situation against a large pharmaceutical company is immensely stressful. |
| Reuters (US) September 8, 2021 Group Sues to Have Hair-loss Drug Propecia Pulled from Market |  | A patient advocacy group has filed a lawsuit seeking to pull Merck & Co’s hair loss drug Propecia and its generic versions from the market, saying federal regulators had failed to act on evidence that it causes depression, erectile dysfunction and, in some cases, suicide. [The PFS] foundation said the [FDA] had unlawfully failed to act despite ‘ample evidence’ that the drug was associated with a higher risk of suicide. |
| Haaretz (Israel) September 18, 2022 Israeli Victims of Propecia Speak Out: ‘I Woke Up Into a Nightmare’ | 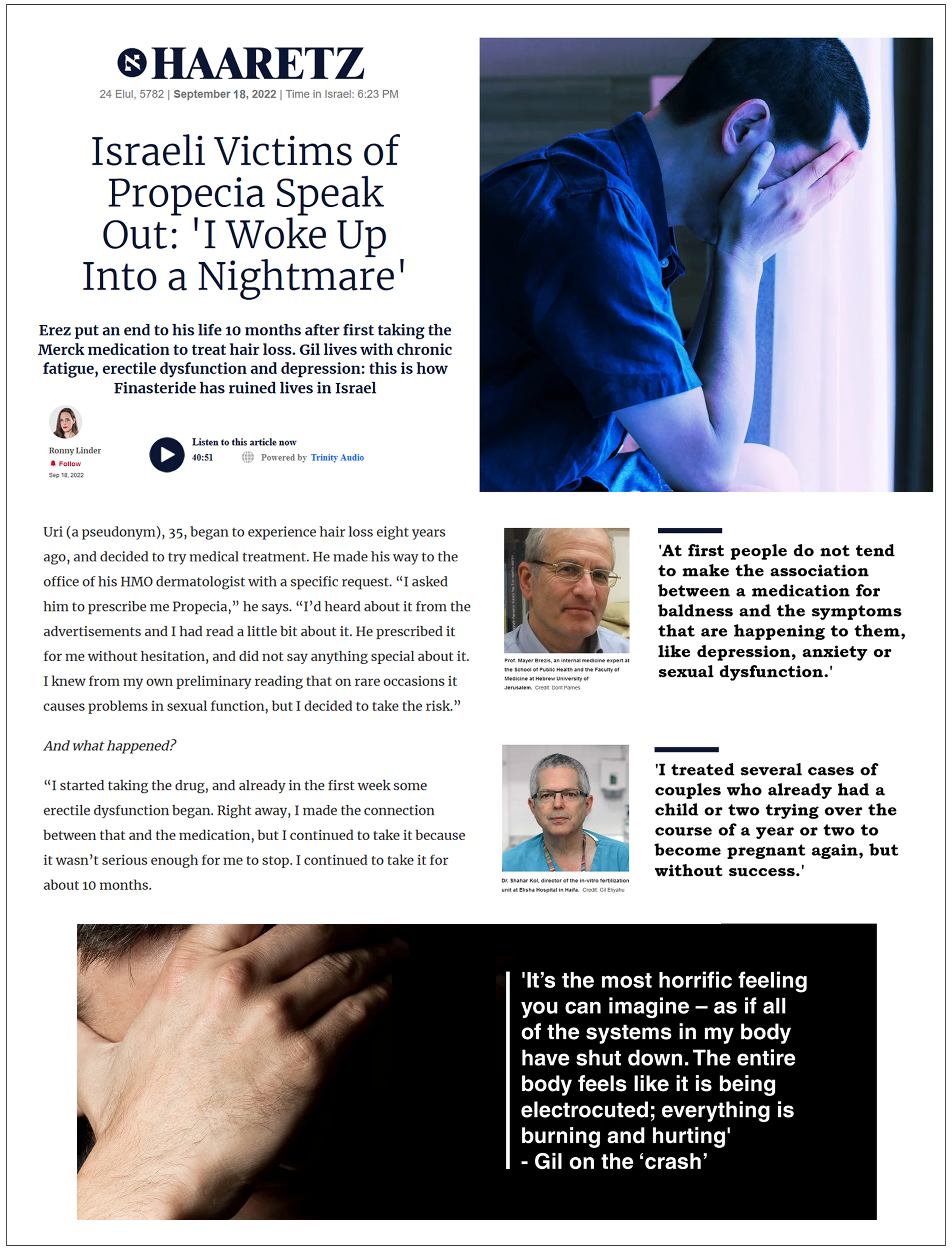 | Jonathan Davies, a medical malpractice attorney [last year] filed a request for a class-action suit against the three pharmaceutical companies that market Propecia and its generic equivalents in Israel, on behalf of those affected. Davies says that after the media reported that he had submitted a class-action suit, he was astounded by the dozens of inquiries he received from men who had been harmed by Propecia. ‘Within days, we received applications from a great deal of young men with side effects of depression and anxiety that significantly affected their quality of life, to the extent that some of these instances justify submitting personal lawsuits, due to the significant damage caused to them,’ said Davies.As early as 2010, a class-action suit against Merck was filed in Israel, alleging that the company had not issued the required warnings of known side effects of impaired fertility and sexual dysfunction, and that there were differences between the inserts in Israel and abroad. The suit ended with a financial settlement in 2018, in which the company paid 5.7 million shekels to the plaintiffs and agreed to update the consumer insert. |
| NDR (Germany) October 25, 2022 Finasteride: Why Is This Hair-loss Drug Still on the Market? |  | Jörg Heynemann is a lawyer specialized in medical law. He’s in court against the large pharmaceutical companies that sell finasteride in Germany… He says it’s difficult to fight Big Pharma. They always use the same strategy…defending themselves by simply saying, “Well, your erectile dysfunction can also stem from the fact that you might’ve smoked a joint or touched a cigarette as a teen. And with that, we’re off the hook.” ‘These procedures are very complex. It must be first determined by a medical report if there is depression present at all, erectile dysfunction, and so on,’ said Heynemann. ‘If at some point the answer is affirmative, then the cause of the symptoms must be researched. And that’s usually the end of the case. The side effects of finasteride are simply too many and there is almost always another conceivable cause for each symptom.’ |








