Stricken US college student corroborates red flag raised to 29,000 pharmacists
Feb. 27, 2023
Dear Friends:
 Spraying finasteride on your scalp instead of ingesting it is no guarantee you won’t develop post-finasteride syndrome (PFS).
Spraying finasteride on your scalp instead of ingesting it is no guarantee you won’t develop post-finasteride syndrome (PFS).
So cautions Germany’s oldest and most widely read pharmaceutical journal.
So, too, an American college senior who says his academic, social and sex life has been decimated by a non-FDA-approved topical-finasteride product from the $2 billion telemed firm Hims & Hers Health (NYSE: HIMS).
Headed off at the press
Earlier this month, Deutsche Apotheker Zeitung (DAZ), which boasts a weekly readership of 29,000 pharmacists, ran a story headlined Is Topical Finasteride a Better Alternative to Treat Alopecia? (English).
The article, by DAZ Drugs & Therapy Editor Desiree Aberle—a pharmacist herself—was prompted by the recent launch of Germany’s first topical-finasteride product, Fynzur for Men, a prescription-only spray from Swiss pharmaceutical company Laboratoires Bailleul. Desiree writes:
In some cases, [side effects], including insomnia or anhedonia, can persist even years after discontinuing finasteride, known as post-finasteride syndrome. This syndrome is highly distressing for those affected, and there are currently no effective treatment options available. Therefore, it is worth considering whether local application to the scalp could reduce the risk of side effects.
Fynzur, she reports, is being touted as probably safer than oral finasteride, based on a 2021 study titled Efficacy and safety of topical finasteride spray solution for male androgenetic alopecia: a phase III, randomized, controlled clinical trial:
The authors of the study conclude that the likelihood of systemic side effects is lower with topical therapy. However, the non-profit organization Post-Finasteride Syndrome Foundation has case reports on PFS with only topical use of finasteride, so patients should still be made aware of this possibility during the consultation.
A little dab’ll do you…in
By way of background, the PFS Foundation has been in business for more than a decade. In that time, we’ve been contacted by some 3,000 desperate PFS patients seeking help. Of those 3,000 patients, 12 (0.4%) have told us they developed the condition after using topical finasteride only.
The first such case arose in 2016, when this young man wrote us:
I’m a 23-year-old who’s moving to NYC and starting a new job this week. I coincidentally just developed what seems to be very debilitating PFS (total lack of sensitivity and erectile function) and am struggling to cope with it in the middle of such a huge life change. I used topical finasteride last year. Now I have trouble just surviving each day, let alone starting a job on Wall Street.
 A year later, in 2017, this 27-year-old from Winchester, UK, wrote:
A year later, in 2017, this 27-year-old from Winchester, UK, wrote:
I’ve been suffering from PFS for over 7 months now. I used it topically for a couple of days. Immediately noticed some ED so quit. The ED didn’t go away and I no longer find women attractive. Lost all motivation and enjoyment in life. I’m desperately seeking some support from someone.
And in 2019, this 28-year-old from Los Angeles wrote:
I started with topical finasteride 0.1% fortified with 6% minoxidil. I’ve never taken the pill form of finasteride (propecia). I discontinued use after 36 days of the topical spray due to side effects. It’s been 3 days since discontinuing and I still have all the side effects. The worst is brain fog and depression, which started after I discontinued use. What originally made me stop were erectile issues, which started about 2-3 weeks into the treatment. I would like to consult with you about your experiences and how to overcome/get back to a normal and if it is even possible?
In 2018, as part of an effort to better serve the ever increasing number of PFS patients knocking on our digital door due to an ever expanding array of health woes, we added a FAQs page to our website. Question 18 read:
Q: Can topical finasteride cause PFS in the same way that oral finasteride does in some men?
A: Most likely, yes. In 2013, Swiss pharmaceutical company Polichem SA conducted a study, published in the Journal of the American Academy of Dermatology and titled Single and repeated dose of finasteride topical solution in subjects with androgenetic alopecia, which showed that topical finasteride is absorbed and lowers serum DHT levels almost to the same degree as the oral medication. Furthermore, we have reports from several patients who used topical finasteride solutions only and still developed PFS symptoms.
Finally, in 2021, after an eleventh young man suffering from “topical PFS” rang us up to report his symptoms, and beg for help, we changed “several” to “many” in the sentence above.
Looking at him head-on, Sumair Ahluwalia bears more of a resemblance to Jake Gyllenhaal than Jude Law. Which is to say, the 21-year-old history major from Chicago shows few, if any, signs that he’s suffering from androgenic alopecia.
“But most men in my family are bald. So growing up, I always assumed I’d wind up the same way,” Sumair, whose parents emigrated from India in 1999, tells us.
“When I was 18, I started noticing a little thinning on the back of my head. Around the same time, I was seeing lots of Hims ads online—like everywhere on Instagram,” he says.
So instead of scheduling an in-person visit with a medical doctor at a brick-and-mortar office, Sumair sought treatment via the telemed.
“There’s no fuss. Hims delivers right to your door,” he adds. “Four months ago, that seemed like the quickest, most discreet solution. In retrospect, it probably ruined my life.”
On Oct. 14 2022, Sumair completed Hims’s online medical assessment for men concerned about hair loss. According to his Hims medical records, he noted having no medical conditions but that he suffers from two mental health conditions: depression and generalized anxiety disorder.
Additionally, he requested topical finasteride and minoxidil spray, due to his mental-health history. At that point in the online assessment, the following message appeared:
Topical finasteride & minoxidil spray is typically recommended to patients with a history of mental health conditions because oral finasteride may cause or worsen symptoms. Oral finasteride has been associated with increased depression, anxiety and suicidality in men younger than 45 using oral finasteride for hair loss. Patients with new or worsening depression or anxiety should stop finasteride use and follow up with their health care provider or a mental health therapist.
Sumair was then directed to complete a Patient Health Questionnaire, to detect the severity of his depression. He noted as well that he’s taking Buspar (buspirone 20 mg), a prescription medication to treat anxiety disorders, and Cymbalta (duloxetine 40 mg), a prescription medication to treat depression and anxiety, and that he had no history of sexual dysfunction.
At that point in the virtual exchange, Charlene Fernandez, a nurse practitioner, typed on Sumair’s chart:
• Clinical photos reviewed. No thinning hair noted.
• Androgenic alopecia prevention, not a candidate for oral finasteride
Nurse Fernandez also wrote directly to Sumair:
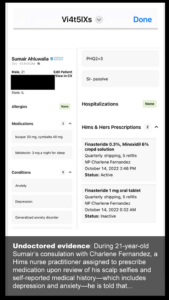 Finasteride can worsen symptoms of depression and anxiety. I have reviewed your medical history and photos and based on the information you provided, I think a strategy focused on preservation with possibly some regrowth will work well. My recommendation for your treatment is the topical finasteride minoxidil spray.
Finasteride can worsen symptoms of depression and anxiety. I have reviewed your medical history and photos and based on the information you provided, I think a strategy focused on preservation with possibly some regrowth will work well. My recommendation for your treatment is the topical finasteride minoxidil spray.
Ten minutes later, however, she shot him a follow-up, which read, in part:
I have prescribed you 30 doses per month of Finasteride Minoxidil Kit 1 mg. Take 1 tablet by mouth daily.
Twenty-one minutes after that, Sumair replied:
I just want to confirm: are you prescribing finasteride pills, or the combo spray? your previous message mentioned the spray but this one mentioned oral finasteride.
To which Nurse Fernandez returned:
My apologies. I’ve contacted customer service to cancel the Finasteride Minoxidil Kit that was erroneously approved… Once the hair loss consultation has been cancelled, you will be able to complete a hair loss consultation requesting Topical 0.3% Finasteride & 6% Minoxidil Spray.
Goodnight, nurse! What’s up, doc?
Eleven days later, shortly after the Topical Finasteride & Minoxidil Spray—a compounded product that Hims notes on its website “has not been approved by the FDA”—shows up in his mailbox, Sumair applied it as directed.
The next day, he was beset by a terrible groin pain like he’d never experienced, which grew worse the day after. And while he didn’t immediately associate it with finasteride, he did email Nurse Fernandez, who replied:
 This is not a typical side effect of Topical 0.3% Finasteride & 6% Minoxidil Spray. I recommend seeking in person evaluation. You can stop the medication in the meantime to see if the symptoms resolve.
This is not a typical side effect of Topical 0.3% Finasteride & 6% Minoxidil Spray. I recommend seeking in person evaluation. You can stop the medication in the meantime to see if the symptoms resolve.
A day later still, Sumair’s groin pain became so intense that he wound up at an urgent care facility, where he was told to quit using topical finasteride. In all, he’d applied the solution for just four days, and never touched it, nor corresponded with Nurse Fernandez, again.
Within a week, his groin pain had subsided, but far worse adverse reactions would soon set in.
“January was horrible,” he says. “I was hit by both sexual and psychological symptoms, including ED, genital shrinkage, insomnia, anhedonia. And the depression, the anxiety—much worse than I’d ever felt before using finasteride.”
Angered at the health crisis Hims had brought about by numerous inferences of topical finasteride being safer than oral, he made an in-person appointment with H Collier Wright, MD, a urologist at Northwestern Medicine.
According to Sumair’s medical records from his visit to Dr. Wright:
In October 2022, he took topical finasteride to prevent hair loss and soon after experience[d] penile pain/irritation. Went to ER for this and was told everything was normal. Subsequently developed ED/low libido… Notes no sex drive… Is concerned he has post finasteride syndrome. Also reports having anxiety/depression… Discussed with patient that post finasteride syndrome is a phenomenon that we fully do not understand or know how to treat.
Also in January, like the hundreds of finasteride-addled men before him, Sumair contacted the PFS Foundation to take part in our Patient Support program. So we dispatched a note to all fellow PFS patients in the Midwest, asking them to contact Sumair. We also compiled a list of all patients who said they’d come down with the condition after using topical finasteride only, and asked them to contact him as well.
Sumair’s physical health and mental health, meanwhile, have not improved since developing full-blown PFS.
He was forced to move out of his dorm at school, and back in with his parents. He hasn’t set foot on campus once this year, and attends all classes via Zoom. Naturally, that has negatively impacted his social life and romantic life.
“Who could’ve imagined that such a tiny amount of liquid—that’s so widely promoted as safe these days—could do such damage,” Sumair says.
“If I’d been properly warned, I never would’ve gone near finasteride. In any form.”
 Anyone living in the US who suffers from PFS should report his or her symptoms to the US FDA. Anyone living outside the US who suffers from PFS should report his or her symptoms to the US FDA as well as to his or her local drug-regulatory authority (DRA), as directed on our Report Your Side Effects page.
Anyone living in the US who suffers from PFS should report his or her symptoms to the US FDA. Anyone living outside the US who suffers from PFS should report his or her symptoms to the US FDA as well as to his or her local drug-regulatory authority (DRA), as directed on our Report Your Side Effects page.
Finally, if you or a loved one are suffering from PFS, and feeling depressed or unstable, please don’t hesitate to contact the PFS Foundation as soon as possible via our Patient Support hotline: social@pfsfoundation.org.
Thank you.
Related news