New UniMi study enumerates pathologies including depression, anxiety, insomnia, and cognitive dysfunction potentially linked to finasteride-induced genetic dysregulation
March 16, 2024
Dear Friends:
Turns out, post-finasteride syndrome may indeed be all in your head—namely, your hypothalamus and hippocampus.
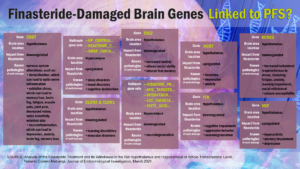 So demonstrates the latest research from the University of Milano (UniMi), which has identified 186 genes in the brain that are either upregulated or downregulated by finasteride, both of which can lead to a host of persistent health woes that closely map many of the most common PFS symptoms.
So demonstrates the latest research from the University of Milano (UniMi), which has identified 186 genes in the brain that are either upregulated or downregulated by finasteride, both of which can lead to a host of persistent health woes that closely map many of the most common PFS symptoms.
Titled Analysis of the Finasteride Treatment and Its Withdrawal in the Rat Hypothalamus and Hippocampus at Whole-Transcriptome Level, and funded by the PFS Foundation, the paper appears in the March issue of the Journal of Endocrinological Investigation, the official journal of the Italian Society of Endocrinology.
Built on Baylor
Roberto Cosimo Melcangi, PhD, Head of the Neuroendocrinology Unit in UniMi’s Department of Pharmacological and Biomolecular Sciences, was prompted to undertake the project by Differential Gene Expression in Post-Finasteride Syndrome Patients, the first-ever study to demonstrate gene-expression differences as a potential cause of sexual dysfunction in PFS patients.
Published in 2021 by Mohit Khera, MD, Director of the Laboratory for Andrology Research at Baylor College of Medicine, that study, also funded by the PFS Foundation, showed that more than 3,700 genes found in the penile tissue of PFS patients were either upregulated or downregulated.
 More distressing still, two of the study’s participants, Daniel M. Stewart, a Professor of Criminal Justice at the University of North Texas and Stephen E. Kenney, a detective sergeant with Georgia’s DeKalb County Police Department, took their own lives soon after data-collection wrapped.
More distressing still, two of the study’s participants, Daniel M. Stewart, a Professor of Criminal Justice at the University of North Texas and Stephen E. Kenney, a detective sergeant with Georgia’s DeKalb County Police Department, took their own lives soon after data-collection wrapped.
“It pains me to no end that I will be causing so much grief,” Det. Kenney wrote in his suicide note. “Please understand that there is no other way for this to end… I can’t live one more day with this condition.”
Prof. Melcangi thus enlisted four members of his team—Silvia Giatti, PhD, Silvia Diviccaro, PhD, Rocco Piazza, MD, and Lucia Cioffi, PhD(c)—to perform a genome-wide analysis of finasteride’s impact on the brain of adult male rats.
Each animal was treated with 1 mg of finasteride per day for 20 consecutive days. Subsequent to that, Team Melcangi performed RNA sequencing analysis in the hypothalamus, an area of the brain that functions as the main link between the endocrine system and nervous system, and in the hippocampus, an area of the brain chiefly responsible for learning, memory, and processing emotions.
The majority of the dysregulation occurred in the hypothalamus, with 171 genes upregulated and 15 downregulated. In the hippocampus, 17 genes were upregulated, and two were downregulated.
“Some of the genes reported to be differentially expressed…suggest a potential link with specific side effects previously observed in [PFS] patients and in the animal model, such as depression, anxiety, disturbance in memory and attention, and sleep disturbance,” writes Prof. Melcangi.
“These data may provide an interesting background for future experiments addressed to confirm the pathological role of these genes in this experimental model, exploring the impact in their signaling pathways, and evaluating possible therapeutic strategies able to counteract their pathological effects,” he concludes.
Persistent ED, too
 On a related note, Team Melcangi’s previous PFS study in an animal model, Exploring Rat Corpus Cavernosum Alterations Induced by Finasteride Treatment and Withdrawal, demonstrated that the type of sexual dysfunction experienced by patients during finasteride treatment differs from the type of sexual dysfunction that persists post-treatment.
On a related note, Team Melcangi’s previous PFS study in an animal model, Exploring Rat Corpus Cavernosum Alterations Induced by Finasteride Treatment and Withdrawal, demonstrated that the type of sexual dysfunction experienced by patients during finasteride treatment differs from the type of sexual dysfunction that persists post-treatment.
In short, ED while on finasteride involves physical alterations in the genitals, while ED after quitting, Prof. Melcangi tells us, “May be related to alterations in neuroendocrine control of sexual desire”—which of course originates in the brain.
Team Melcangi has been investigating PFS for more than a decade, publishing an average of one paper per year on the condition:
• Neuroactive steroid levels are modified in cerebrospinal fluid and plasma of PFS… The Journal of Sexual Medicine, 2013
• Adverse effects of 5α-reductase inhibitors: : What do we know, don’t… Reviews in Endocrine and Metabolic Disorders, 2015
• Patients treated for male pattern hair with finasteride show, after discontinuation… Journal of Steroid Biochemistry, 2015
• Effects of subchronic finasteride treatment and withdrawal on neuroactive steroid levels and… Neuroendocrinology, 2016
• Neuroactive steroid levels and psychiatric and andrological features in PFS patients Journal of Steroid Biochemistry, 2017
• Treatment of male rats with finasteride, an inhibitor of 5alpha-reductase enzyme, induces… Psychoneuroendocrinology, 2019
• Altered methylation pattern of the SRD5A2 gene in cerebrospinal fluid of PFS patients: a pilot… Endocrine Connections, 2019
• Alterations of gut microbiota composition in PFS patients: a pilot study Journal of Endocrinological Investigation, 2020
• Post-finasteride syndrome: An emerging clinical problem Neurobiology of Stress, 2020
• Three-Dimensional Proteome-Wide Scale Screening for the 5‑AR Inhibitor Finasteride… Journal of Medicinal Chemistry, 2021
• Gut Inflammation Induced by Finasteride Withdrawal: The Therapeutic Affect of Allopregnanolone in… Biomolecules, 2022
• Exploring Rat Corpus Cavernosum Alterations Induced by Finasteride Treatment and Withdrawal Andrology, 2023
• PFS and Post-SSRI Sexual Dysfunction: Two Clinical Conditions Apparently Distant… Frontiers in Neuroendocrinology, 2024
Finasteride was originally developed by Merck & Co., Inc., and first approved by the US Food and Drug Administration in 1993 as Proscar (5 mg, for enlarged prostate), and again in 1997, as Propecia (1 mg, for hair loss).
In June 2021, Merck spun off its Organon subsidiary as an independent public company (NYSE: OGN). Founded in the Netherlands in 1923, Organon bills itself as a “global health care company dedicated to making a world of difference for women, their families and the communities they care for.”
 Among the Merck products Organon acquired in the deal were Proscar and Propecia. To report adverse events for either finasteride product, call the Organon Service Center at (844)674-3200, or email Service_Center@Organon.com.
Among the Merck products Organon acquired in the deal were Proscar and Propecia. To report adverse events for either finasteride product, call the Organon Service Center at (844)674-3200, or email Service_Center@Organon.com.
Anyone living in the US who suffers from PFS should also report his or her symptoms to the US FDA. Anyone living outside the US who suffers from PFS should report his or her symptoms to the US FDA as well as to his or her local DRA, as directed on our Report Your Side Effects page.
If you or a loved one are suffering from PFS, and feeling depressed or unstable, please don’t hesitate to contact the PFS Foundation as soon as possible via our Patient Support hotline: social@pfsfoundation.org
Thank you.
