Following new safety review, Health Canada working with finasteride manufacturers to strengthen suicidality warning
March 27, 2023
Dear Friends:
 Although the US Food and Drug Administration rarely mentions PFS, its database of side effects speaks volumes about the condition’s epidemiology.
Although the US Food and Drug Administration rarely mentions PFS, its database of side effects speaks volumes about the condition’s epidemiology.
Out of control
According to new research based in part on the agency’s Adverse Event Reporting System (FAERS), finasteride use is, on average, 23 times as likely to precipitate depression and anxiety as two control medications: Inderal, a beta-blocker linked to depression, and Spironolactone, a potassium-sparing diuretic used to treat alopecia.
By the same measures, finasteride use is nine times as likely to bring about insomnia, fatigue, suicidality, and suicide.
Titled Neuropsychiatric Reactions to Finasteride: Nocebo or True Effect? the 1,100-word paper by Mayer Brezis, MD, Professor of Medicine Emeritus at the Hebrew University of Jerusalem, was published last month in the Journal of Basic and Clinical Pharmacy.
Dr. Brezis writes:
Reports of adverse reactions increasingly suggest that finasteride may cause depression, anxiety, suicidality and sexual dysfunction, even after discontinuation of the drug. On the other hand, some publications have claimed that this could represent simulated reporting of a nocebo effect. In the present paper, we…demonstrate remarkably disproportionate safety signals for finasteride.
 Furthermore, the rise in neuropsychiatric reactions to finasteride over the last decade concurs with a striking increase in suicides reported to the FDA in relationship to this medication.
Furthermore, the rise in neuropsychiatric reactions to finasteride over the last decade concurs with a striking increase in suicides reported to the FDA in relationship to this medication.
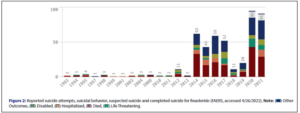 Via FAERS, Dr. Brezis accessed suicidality data for finasteride from 1993 through 2021, which include reports of suicide attempts, suicidal behavior, suspected suicide and completed suicide, the outcomes of which where: died, disabled, hospitalized, life threatening, or other.
Via FAERS, Dr. Brezis accessed suicidality data for finasteride from 1993 through 2021, which include reports of suicide attempts, suicidal behavior, suspected suicide and completed suicide, the outcomes of which where: died, disabled, hospitalized, life threatening, or other.
The absolute number of such reports peaked in 2020, at 95, falling just 4% in 2021, to 91. From 2019 to 2020, however, the number of such incidents rose 375%.
Even more striking is the decade-over-decade data. In 2010, FAERS contained just two suicidality reports for finasteride, which translates into a rise of 4,650% by 2020. And in 2001, FAERS contained just one suicidality report for finasteride, translating into a rise of 9,000% by 2021.
Dr. Brezis also notes:
Since suicide has not been associated with a nocebo effect, it seems likely that we are facing real and serious adverse effects on mood from finasteride. Increased reporting could relate to enhanced awareness and not to a nocebo effect.
Being branded crazy nothing new to PFS patients
Within the PFS Foundation’s database of some 3,000 proactive case reports, about 400 (13%) have complained about doctors characterizing the condition as psychosomatic. For instance:
In 2019, JB, then 22, from Los Angles, CA, wrote:
I started finasteride a month ago and just haven’t felt like myself at all… There’s been an uptick in suicidal ideation, as well as anxiety. My heart has felt cold and ungiving of emotion. Things I normally live for, like livestreaming with fans, seem to hold little appeal to me… I haven’t been getting erections often… Doctors keeps telling me virtually no one suffers from PFS…that it’s all in my head.
In 2017, AA, then 26, from Setúbal, Portugal, wrote:
I don’t smoke, no alcohol, no drugs, no meds, practice swimming etc. I work as a respiratory physiologist… I took finasteride 1 mg daily for two years. Now I experience side effects like prostatitis, weak urinary flow, numb penis, genital atrophy, zero libido, extreme brain fog, extreme extreme fatigue, insomnia, and suicidal thoughts. I had some consultations with urologists, endocrinologists, dermatologists, etc., and all of them just ignored my condition. They said it’s all in my head… I can’t wait any longer for a cure. I’m going to kill myself in a short term.
Fanning the flames of such apathy among health care providers, and distress among patients, have been two research papers, both by dermatologists, published around the same time as those complaints:
• Investigation of the Plausibility of 5-Alpha-Reductase Inhibitor Syndrome. Skin Appendage Disorders: Raymond Fertig, MD, University of Miami Miller School of Medicine, 2016. Dr. Fertig writes:
A significant placebo/nocebo effect has been documented among patients informed about possible side effects of finasteride and this may explain the high prevalence of reported sexual dysfunction including persistent dysfunction in subjects participating in Internet groups and blogs.
 • Post-Finasteride Syndrome: An Induced Delusional Disorder with the Potential of a Mass Psychogenic Illness? Skin Appendage Disorders: Ralph M. Trüeb, MD, University of Zurich, 2019. Dr. Trüeb writes:
• Post-Finasteride Syndrome: An Induced Delusional Disorder with the Potential of a Mass Psychogenic Illness? Skin Appendage Disorders: Ralph M. Trüeb, MD, University of Zurich, 2019. Dr. Trüeb writes:
The concept of PFS has emerged from reports of non-dermatologists, neuroendocrinological research and reflections, and uncontrolled studies of low quality and with a strong bias selection, while a significant nocebo effect among patients informed about possible side effects of finasteride is recognized.
It’s all in your head, insinuates telemed
More recently, the world’s fifth largest telemedicine firm has furthered the notion that PFS may merely be the result of a nocebo effect.
Among the digital consumer content currently available from Hims & Hers Health (NYSE: HIMS) is a page titled Post Finasteride Syndrome: What is It? The 2,700-word blog post, written by Hims’ editorial team, and “medically reviewed” by nurse practitioner Kate Hagerty, was last updated in October 2021. Some pertinent passages:
• Within the medical community, there’s debate about whether post-finasteride syndrome is a real medical condition or something that’s largely psychological… Experts believe that the symptoms commonly described as post-finasteride syndrome could be caused by several different factors.
• The first of these is the nocebo effect. This is a psychological phenomenon in which a person’s negative expectations about a specific medication or procedure may cause them to experience a negative symptom.
• A second factor that may explain the symptoms of [PFS] is existing mental illness… Researchers have theorized that the reported symptoms of [PFS] may not be caused by finasteride itself, but just incidentally associated with finasteride use.
 Feds finally feeling patients’ pain
Feds finally feeling patients’ pain
For all the finger-pointing that could be directed at the FDA vis-à-vis its failure to warn the public of aberrant spikes in suicide stemming from a drug it approved 30 years ago, we’ll instead look forward to stricter regulations in the not-too-distant future.
Because just 10 days ago, in its first-ever public reference to the condition, the agency published the topics of its upcoming Patient Listening Sessions, one of which is post-finasteride syndrome.
Instituted in 2018, FDA Patient Listening Sessions (PLS):
• Are small, informal, non-regulatory, non-public teleconference meetings that allow participants to connect with FDA staff first-hand.
• Are about patient experiences, perspectives, and needs related to their health or a disease, and not about specific medical products (drug, biologic, or device).
• Give the FDA an opportunity to connect with under-represented communities.
Among their goals are:
• To educate FDA review staff about diseases, conditions, and health-related experiences and perspectives that are important to patients, caregivers, advocates, and community representatives.
• To inform regulatory decision-making, e.g., for a specific product or application, benefit/risk trade-offs, developing guidances.
• To help provide a starting point to guide or accelerate early-stage research and development, e.g., helping to identify endpoints and new outcome assessment tools.
After each PLS, which can be either FDA-requested or patient-led, patient-group managers are encouraged to develop a summary of the event. Once that summary is reviewed by the FDA, it’s posted on the agency’s PLS Summaries page.
Stay tuned for the PFS summary, which we trust will put our federal government one step closer to acknowledging that post-finasteride syndrome is caused by, yes, finasteride.
Canada cracks down further on fin
In stark contrast to the US, Canada has been actively monitoring finasteride’s potential link to suicidality for more than a decade now—and making its findings public. Health Canada (HC), the nation’s drug-regulatory authority (DRA), completed its first finasteride-safety review in 2012, followed by a second in 2015.
 But it wasn’t until its third review, in 2019, that HC detected an epidemiological signal strong enough to take action; namely, updating the finasteride product monograph (aka, in the US, product label) to include a risk of suicidal ideation.
But it wasn’t until its third review, in 2019, that HC detected an epidemiological signal strong enough to take action; namely, updating the finasteride product monograph (aka, in the US, product label) to include a risk of suicidal ideation.
Undertaken in 2022, its purpose “was to consider recent information and determine if additional measures were warranted.”
Findings are delineated, in part, as follows:
• Health Canada reviewed 401 cases…of suicide, suicidal ideation and/or self-injury in patients using finasteride from the Canada Vigilance database. Of the 401 cases, 25…met the criteria for further assessment to determine if there was a link between the use of finasteride and suicide, suicidal ideation and self-injury.
• Of the 25 cases, 23…were found to be possibly linked to the use of finasteride.
• In 17 of the 25 cases…patients were 40 years of age or younger and taking finasteride for male pattern hair loss.
• [HC] also reviewed 16 publications in the scientific literature. There is a growing body of scientific evidence regarding the association between the use of finasteride and the risks of suicide, suicidal ideation and self-injury.
As such, much in line with recent measures by French counterpart ANSM, HC reports that it:
• is working with the manufacturers to update the product safety information…for finasteride-containing products to strengthen the warning statements on the risks of suicidal ideation and self-injury, and to include information about patient screening for psychiatric risk factors prior to starting treatment, as well as continuous patient monitoring during and after stopping treatment.
• will also inform healthcare professionals about this update through a Health Product InfoWatch communication.
(For information on how other nations have been monitoring finasteride adverse events, disclosing risks to the public, and regulating the medication, see our PFS Global Warning Map.)
Reuters to the rescue
 The media report HC says triggered its fourth safety review is one that grew out of a year-long investigation into malfeasance by finasteride manufacturer Merck & Co. (NYSE: MRK).
The media report HC says triggered its fourth safety review is one that grew out of a year-long investigation into malfeasance by finasteride manufacturer Merck & Co. (NYSE: MRK).
In September 2019, Reuters ran a 3,900-word investigative report headlined, Court let Merck hide secrets about a popular drug’s risks. Written by Dan Levine, the story uncovered testimony by former Merck executives in the US Propecia litigation suggesting that the company downplayed finasteride’s side effects during clinical trials.
A day after that story broke, the news agency filed a motion in US Federal Court to unseal documents filed in the Propecia litigation, noting, “This is a case of tremendous importance that has been sealed without on-the-record findings explaining that sealing. The First Amendment precludes such an outcome.”
In January 2021, US Magistrate Judge Peggy Kuo approved Reuters’ motion, clearing the way to make documents in the Propecia litigation public.
Ten days after that, in a story headlined Merck anti-baldness drug Propecia has long trail of suicide reports, records show, Reuters reported:
Merck & Co. and US regulators knew about reports of suicidal behavior in men taking… Propecia when they decided not to warn consumers of those potential risks in a 2011 [label] update… Since the 2011 decision on the warning, the FDA has received more than 700 reports of suicide and suicidal thoughts among people taking…the drug. Those included at least 100 deaths. Before that, in the first 14 years the drug was on the market, the agency received 34 such reports, including 10 deaths.
And with that, Canada began digging, yet again, into the PFS epidemic.
 Anyone living in the US who suffers from PFS should report his or her symptoms to the US FDA. Anyone living outside the US who suffers from PFS should report his or her symptoms to the US FDA as well as to his or her local drug-regulatory authority (DRA), as directed on our Report Your Side Effects page.
Anyone living in the US who suffers from PFS should report his or her symptoms to the US FDA. Anyone living outside the US who suffers from PFS should report his or her symptoms to the US FDA as well as to his or her local drug-regulatory authority (DRA), as directed on our Report Your Side Effects page.
Finally, if you or a loved one are suffering from PFS, and feeling depressed or unstable, please don’t hesitate to contact the PFS Foundation as soon as possible via our Patient Support hotline: social@pfsfoundation.org.
Thank you.
