Las autoridades reguladoras de medicamentos revelan los peligros potenciales, la disfunción sexual y el suicidio durante una década, incluiendo
Solo superado por “¿Existe una cura para el PFS?” la pregunta más común que nos surge es: “¿Cómo es posible que este medicamento siga en el mercado?” Los que preguntan son (a) hombres jóvenes que se enfrentan a la realidad de que un remedio «seguro y eficaz» para la caída del cabello los ha mutilado, tal vez de por vida, o (b) los seres queridos de un paciente con PFS que recientemente amenazó, intentó o suicidio consumado. Nuestra respuesta inicialmente fue: “Nos gana”. Porque nos gusta pensar que cualquier persona astuta que haya examinado la evidencia que se encuentra aquí (bibliografía médica, datos epidemiológicos, testimonios de médicos, súplicas de pacientes, cobertura de los medios y malversación corporativa) concluiría que la autorización de comercialización de la finasterida debe cancelarse de inmediato. Luego explicamos que, desde el lanzamiento de la droga en 1993, ha habido una serie de medidas por parte de las autoridades federales reguladoras de drogas para advertir al público de sus muchos peligros potenciales. La Agencia Sueca de Productos Médicos puso las cosas en marcha en 2008 al ordenar que Merck agregara la disfunción sexual persistente y otras reacciones adversas a medicamentos (RAM) a la etiqueta de finasterida. Durante los siguientes 13 años, agencias que van desde la FDA de los EE. UU. hasta la FDA de Arabia Saudita hicieron lo mismo con ADR que van desde la ansiedad hasta el suicidio consumado. Esperamos que esta creciente protesta mundial culmine algún día con la eliminación de la finasterida del mercado. Mientras tanto, para ayudarlo a navegar por el historial de farmacovigilancia cada vez más complejo del medicamento, hemos identificado 56 países que han emitido advertencias. A continuación se encuentran las fuentes de esas advertencias, junto con los principales síntomas de PFS incluidos en ellas. Para obtener una gama completa de síntomas de PFS informados, consulte nuestra página Acerca de PFS.
Si conoce otras naciones que han emitido advertencias de PFS y/o desea sugerir fuentes adicionales para complementar las que se enumeran a continuación, comuníquese con Philip Roberts. Esta página se publicó el 27 de junio de 2022. Para leer el anuncio de lanzamiento, haga clic aqui.
Noticias relacionadas: SPF es ‘claramente un problema’, ‘garantiza una intervención’ y es ‘inapropiado descartarlo’, acuerdo a una nueva investigación de la USC
Naciones la referencia SPF: 56
| Argentina National Pharmacovigilance System PFS symptoms referenced
|  | Cases of depression and, more rarely, suicidal ideation have been observed in men treated with finasteride 1 mg for hair loss. The risk of depression is also associated with finasteride… Patients and healthcare professionals are advised that any change in mood should lead to treatment discontinuation.—International and National News on Drug Safety: October 2017 (English) |
| Australia Therapeutic Goods Administration PFS symptoms referenced
|  | [T]here is the possibility that finasteride use may result in Post Finasteride Syndrome... [D]own scheduling finasteride to Schedule 3 would set a dangerous precedent for Category X medications, and the potential risks from broader access under Schedule 3 significantly outweigh the benefits.—Interim Decision in Relation to Finasteride: June 2019[D]epression (feelings of severe sadness and unworthiness) including suicidal thoughts. These are uncommon side effects that have been reported with [finasteride].—Propecia Consumer Medicine Information: January 2021 |
| Austria Federal Office for Safety in Healthcare PFS symptoms referenced
|  | In patients treated with [finasteride], mood changes such as depressed mood, depression and, less commonly, suicidal thoughts were reported. If you notice any of these symptoms, consult your doctor immediately.—Change in the permits for the marketing of finasteride: December 2017 (English)Mood changes and depression: In patients treated with Sandoz finasteride, mood changes such as depressed mood, depression and, less commonly reported, suicidal thoughts… Side effects can occur [including] inability to get an erection that persists after stopping treatment, decreased sexual drive that persists after stopping treatment.—Sandoz finasteride 5 mg package leaflet: February 2020 (English) |
| Azerbaijan Ministry of Health of Pharmacological and Pharmacopoeial Expert Council PFS symptoms referenced
|  | The following side effects have been reported with post-marketing use of finasteride: Hypersensitivity reactions, pruritus, edema of the face and lips, pain in the testicles, palpitations, elevated liver enzymes, depression. In some individuals…these symptoms were persistent.—Instructions for the use of finasteride 5 mg: 2020 |
| Belarus Center for Examinations and Tests in Health Service PFS symptoms referenced
|  | [D]epressed mood, depression and, less commonly, suicidal thoughts have been reported in patients taking finasteride. Patients should be evaluated for psychiatric disorders and, if they occur, the patient should seek medical help… Reproductive system and breast disorders [include] sexual dysfunction that may persist after treatment is stopped.—Instructions for the use MIC finasteride 5 mg: May 2021 (English) |
| Belgium Federal Agency for Medicines and Health Products PFS symptoms referenced
|  | New data, analyzed by the European Medicines Agency, has concluded that the risks of mood disorders, suicidal ideation and depression are possible while taking finasteride at 1 mg/day… Treatment with finasteride should be discontinued in the event of any psychiatric symptoms. It is important that patients are informed of these risks, since no leaflet accompanies the custom prescriptions [i.e., those prepared by pharmacists per doctors' specifications] of finasteride at 1mg.—Federal Agency for Medicines and Health Products News: November 2017 (English)Additional sources —Summary of product characteristics for EG finasteride 5 mg: March 2020 (English) —EG finasteride 5 mg package leaflet: March 2020 (English) |
| Brazil National Health Surveillance Agency PFS symptoms referenced
|  | [F]inasteride, when applied topically, has systemic absorption, thus leading to the same side effects when used orally. [The Technical Advisory Committee on Cosmetics] recommends: Prohibit the use of finasteride in cosmetic products.—Technical Opinion No. 4: February 2002 (English)Patients should also be aware of the risk of sexual dysfunction...during treatment with finasteride. Patients should also be informed that, based on individual patient case reports, sexual dysfunction may also persist for more than 10 years after discontinuation of treatment.—Merck SA Letter to Health Professionals: May 2019 (English) |
| Bulgaria Bulgarian Drug Agency PFS symptoms referenced
|  | Mood swings such as depressive mood, depression and, rarely, suicide thoughts, have been reported in patients treated with Accord finasteride 1 mg. If you have any of these symptoms, stop taking Accord finasteride…and contact your doctor as soon as possible… Possible adverse reactions: permanent erectile dysfunction after stopping the treatment; permanent decrease in libido after stopping the treatment; permanent ejaculation problems after stopping the treatment.—Accord finasteride 1 mg package leaflet: June 2019 (English) |
| Canada Health Canada PFS symptoms referenced
|  | New information regarding the risk of muscle-related disorders has been added to the Post-Market Adverse Drug Reactions and Consumer Information sections of the Canadian product monographs for Proscar and Propecia…Rare cases of muscle-related disorders, such as rhabdomyolysis, myopathy, myalgia, myasthenia, and creatine kinase elevation, have been reported in patients treated with finasteride.—Health Product InfoWatch: Proscar and Propecia: June 2018Health Canada reviewed the risk of suicidal thoughts and/or behaviours (suicidal ideation) with Proscar and Propecia (finasteride) use. The safety review was triggered because of Canadian and international reported cases of suicidal ideation and self-injury. Health Canada's review concluded that there may be a link between Proscar and Propecia...and the risk of suicidal ideation.—Assessing the potential risk of suicidal thoughts and/or behaviour: February 2019This safety review evaluated the risk of suicide, suicidal ideation and self-injury associated with the use of finasteride-containing products. [We] found a possible link between the use of finasteride and the risks of suicidal ideation and self-injury... ealth Canada is working with the manufacturers to update the Canadian product monographs for finasteride-containing products to strengthen the warning statements on the risks of suicidal ideation and self-injury, and to include information about patient screening for psychiatric risk factors prior to starting treatment, as well as continuous patient monitoring during and after stopping treatment.—Summary Safety Review - Finasteride-containing products: January 2023Like any medicine, Propecia may cause side effects.Common side effects:• less desire to have sex • difficulty in achieving an erection. • problems with ejaculation... In clinical studies, these side effects disappeared in men who stopped taking Propecia and in most men who continued treatment. In general use, the following have been reported infrequently: • breast enlargement (swelling) and/or tenderness; • depression; • decrease in sex drive that continued after stopping the medication; • allergic reactions including rash, itching, hives, and swelling of the lips, tongue, throat and face; • muscle injury, muscle pain, muscle weakness, abnormal test results (CK elevation); • problems with ejaculation that continued after stopping the medication; • testicular pain; • blood in semen • difficulty in achieving an erection that continued after stopping the medication; • male infertility and/or poor quality of semen... • male breast cancer; • changes in mood, which can include suicidal thoughts. —Organon Canada Propecia Product Monograph: April 2021 Propecia may cause changes in mood including extreme sadness... injuries from hurting yourself on purpose... and thoughts of suicide.... These mental health problems may continue even after you stop treatment.Behaviour and mood changes:• There have been reports that Propecia may cause changes in mood including extreme sadness (depression), injuries from hurting yourself on purpose (self-harm injury), and thoughts of suicide (suicidal ideation). These mental health problems may continue even after you stop treatment. • Tell your healthcare professional if you have had these behaviour and mood changes before. They should check your mental health before, during and after your treatment with Propecia. • If you feel sad, want to hurt yourself, or end your own life or if others around you notice changes in your behaviour, get medical help right away. —Organon Canada Propecia Product Monograph: November 2023 |
| China National Medical Products Administration PFS symptoms referenced
|  | New information regarding the risk of muscle-related disorders has been added to the Post-Market Adverse Drug Reactions and Consumer Information sections of the Canadian product monographs for Proscar and Propecia…Rare cases of muscle-related disorders, such as rhabdomyolysis, myopathy, myalgia, myasthenia, and creatine kinase elevation, have been reported in patients treated with finasteride.—Health Product InfoWatch: Proscar and Propecia: June 2018Health Canada reviewed the risk of suicidal thoughts and/or behaviours (suicidal ideation) with Proscar and Propecia (finasteride) use. The safety review was triggered because of Canadian and international reported cases of suicidal ideation and self-injury. Health Canada's review concluded that there may be a link between Proscar and Propecia...and the risk of suicidal ideation.—Assessing the potential risk of suicidal thoughts and/or behaviour: February 2019This safety review evaluated the risk of suicide, suicidal ideation and self-injury associated with the use of finasteride-containing products. [We] found a possible link between the use of finasteride and the risks of suicidal ideation and self-injury... ealth Canada is working with the manufacturers to update the Canadian product monographs for finasteride-containing products to strengthen the warning statements on the risks of suicidal ideation and self-injury, and to include information about patient screening for psychiatric risk factors prior to starting treatment, as well as continuous patient monitoring during and after stopping treatment.—Summary Safety Review - Finasteride-containing products: January 2023Like any medicine, Propecia may cause side effects.Common side effects:• less desire to have sex • difficulty in achieving an erection. • problems with ejaculation... In clinical studies, these side effects disappeared in men who stopped taking Propecia and in most men who continued treatment. In general use, the following have been reported infrequently: • breast enlargement (swelling) and/or tenderness; • depression; • decrease in sex drive that continued after stopping the medication; • allergic reactions including rash, itching, hives, and swelling of the lips, tongue, throat and face; • muscle injury, muscle pain, muscle weakness, abnormal test results (CK elevation); • problems with ejaculation that continued after stopping the medication; • testicular pain; • blood in semen • difficulty in achieving an erection that continued after stopping the medication; • male infertility and/or poor quality of semen... • male breast cancer; • changes in mood, which can include suicidal thoughts. —Organon Canada Propecia Product Monograph: April 2021 |
| Colombia National Institute for Food and Drug Surveillance PFS symptoms referenced
|  | Rare cases…of depression, suicidal thoughts and sexual dysfunction have been identified in the UK in men being treated with finasteride… Patients treated with finasteride should be warned about the possibility of developing depressive disorders, as well as sexual dysfunction, while taking this medicine.—Finasteride Information for Health Professionals: November 2017 (English) |
| Croatia Agency for Medicinal Products and Medical Devices PFS symptoms referenced
|  | The CMDh considered that the changes recommended for finasteride 1 mg tablets…should additionally be part of the single assessment outcome of this PSUR procedure. Further information…will be published on the [European Medicines Agency] website.—Highlights from the CMDh meeting: April 2017[T]he product information of medicinal products containing 5 mg finasteride…should be updated to include a warning on depression and suicidal ideation. [The Marketing Authorisation Holders]…should closely monitor events of suicide, suicidality and self-injury…The MAHs should also closely monitor events of penile size reduced and testicular atrophy. In addition, the MAHs should provide a cumulative review of anxiety [and] a cumulative review of reports describing sexual dysfunction-related events reported in conjunction with psychiatric disorder events… Finally, the MAHs…should provide a cumulative review of psychiatric disorders persisting after finasteride discontinuation.—EMA Pharmacovigilance Risk Assessment Committee on Finasteride: April 2017 |
| Cyprus Republic of Cyprus Ministry of Health PFS symptoms referenced
|  | Mood changes and depression: In patients treated with Accord finasteride, mood changes such as depressed mood, depression and, less commonly reported, suicidal thoughts… Side effects can occur... inability to get an erection that persists after stopping treatment, decreased sexual drive that persists after stopping treatment.—Accord finasteride 5 mg package leaflet: December 2020 (English) |
| Czechia State Institute for Drug Control Medicinal Products Database PFS symptoms referenced
|  | Mood swings, including depressed mood, depression and, rarely, suicidal ideation, have been reported in patients treated with finasteride 5 mg. Patients should be monitored for psychiatric symptoms and, if they do occur, patients should be advised to consult a physician… Psychiatric disorders [include] decreased libido, which continues after treatment, anxiety… Reproductive system [disorders include] sexual dysfunction…which continues after treatment.—Aurovitas finasteride 5 mg package leaflet: September 2019 (English) |
| Denmark Danish Medicines Agency PFS symptoms referenced
|  | The [Danish Medicines Agency], has been contacted by several citizens who have experienced psychiatric adverse reactions in connection with a longer course of treatment with finasteride (1 mg) for hair loss… The issue was discussed at the last meeting in the European Pharmacovigilance Risk Assessment Committee, PRAC, which resolved to place a warning in the product information. The warning will describe that there have been reports of depression, mood changes and suicidal thoughts in treatment with finasteride for hair loss.—Danish Pharmacovigilance Update: May 2017Additional source —Finasteride 1 mg package leaflet: November 2019 (English) |
| Estonia State Medicines Agency PFS symptoms referenced
|  | Mood swings such as depressive mood, depression and, rarely, suicide thoughts, have been reported in patients treated with Accord finasteride... If you have any of these symptoms, stop taking Accord finasteride…and contact your doctor as soon as possible… Possible adverse reactions: permanent erectile dysfunction after stopping treatment; permanent decrease in libido after stopping treatment; permanent ejaculation problems after stopping treatment.—Accord finasteride 5 mg package leaflet: December 2021 (English) |
| Finland Finnish Medicines Agency PFS symptoms referenced
|  | Mood swings such as depressive mood, depression and, rarely, suicide thoughts, have been reported in patients treated with Orion finasteride... If you have any of these symptoms, stop taking Orion finasteride…and contact your doctor as soon as possible… Possible adverse reactions: permanent erectile dysfunction after stopping treatment; permanent decrease in libido after stopping treatment; permanent ejaculation problems after stopping treatment.—Orion finasteride 5 mg package leaflet: July 2019 (English) |
| France • National Agency for the Safety of Medicine and Health Products • Network of Regional Pharmacovigilance Centers PFS symptoms referenced
|  | Patients have reported sexual disorders when using finasteride 1 mg. These may include erection disorders, ejaculation disorders…testicular pain, a decrease in libido, as well as male infertility problems and/or poor semen quality... Patients have also reported mental disorders, including anxiety, depression [and] suicidal thoughts that could lead to suicide. Symptoms of depression may include constant sadness, depression, loss of interest and pleasure, difficulty concentrating and remembering, reduced energy or fatigue (abnormal or even intense) and sleep disorders. All of these disorders can have an impact on social and professional life. Sexual and psychological disorders may persist after stopping treatment for an indefinite period.—ANSM News: Risks of Taking Finasteride 1 mg: July 2022 (English)Remember that finasteride 1 mg has a hormonal action and can cause mental disorders and sexual disorders… Given the nature of the adverse effects, in particular those of a psychological nature, it is advisable to schedule a follow-up consultation within three months after initiation of treatment, to assess its tolerance, then regularly (for example, every 6 months) during the treatment.—ANSM News: Information for Healthcare Professionals about Finasteride 1 mg: July 2022 (English)In our ongoing efforts to promote proper and safe use of finasteride 1 mg, we are making available…new tools designed in collaboration with the Association for Aid to the Victims of Finasteride [including] a “step-by-step” video, from the Network of Regional Pharmacovigilance Centers, to facilitate the reporting of adverse reactions associated with this drug.—ANSM News: Finasteride 1 mg for the Treatment of Early-stage Hair Loss: July 2022 (English)Additional sources —Finasteride 1 mg (Propecia and generics): Added warning statements on boxes to reinforce information on adverse effects: November 2022 (English) —Centres Régionaux de Pharmacovigilance: Reporting ADRs (video, French only): July 2022 —ANSM News: Finasteride 1 mg and Hair Loss: July 2022 (English) —Letter to health professionals regarding finasteride's potential risks: November 2019 (English) —Reminder regarding the risks of psychiatric disorders: February 2019 (English) |
| Germany • Federal Institute for Drugs and Medical Devices • Paul Ehrlich Institute PFS symptoms referenced
|  | Patients should be aware of the risk of sexual dysfunction (including erectile dysfunction, ejaculation disorder, and decreased libido) when starting finasteride therapy. Patients should also be informed that, based on individual patient case reports, sexual dysfunction may persist for more than 10 years after discontinuation of the therapy. Patients should also be advised that mood changes (including depressive mood, depression and suicidal thoughts) have been reported… On the recommendation of the European Medicines Agency, 'anxiety' is included as a new side effect.—Red Hand Letter on finasteride: July 2018 (English)[The US Food and Drug Administration]'s side effects database…showed that use of 1 mg finasteride in the indication of androgenetic alopecia could be associated with the risk of persistent sexual dysfunction due to disproportionate reporting rates. [S]tudies also indicated that sexual dysfunction could persist after discontinuation of finasteride [ranging] from months to years. The [Federal Institute for Drugs and Medical Devices] is aware of a case from Germany in which the reported sexual dysfunction persisted for more than 10 years… A total of 38 case reports on sexual dysfunction when taking finasteride are available in Germany, for which the outcome is given as “not recovered/not resolved.”—Safety Bulletin on depression and sexual dysfunction: September 2018 (English) |
| Greece National Organization for Medicines PFS symptoms referenced
|  | Mood swings such as depressive mood, depression and, rarely, suicide thoughts, have been reported in patients treated with Avelid finasteride... If you have any of these symptoms, stop taking Avelid finasteride…and contact your doctor as soon as possible… Possible adverse reactions: permanent erectile dysfunction after stopping treatment; permanent decrease in libido after stopping treatment; permanent ejaculation problems after stopping treatment.—Avelid finasteride 1 mg package leaflet: November 2019 (English) |
| Hungary National Institute of Pharmacy and Nutrition PFS symptoms referenced
|  | Possible adverse reactions[:] permanent erectile dysfunction after stopping treatment, permanent decrease in libido after stopping treatment, permanent ejaculation problems after stopping treatment.—Sandoz finasteride 1 mg package leaflet: February 2020 (English)Psychiatric disorders: Depression, decreased libido that persists after stopping treatment, anxiety. Reproductive system and breast disorders: Testicular pain, erectile dysfunction that persists after stopping treatment; male infertility and/or poor sperm quality.—Sandoz finasteride 5 mg summary of product characteristics: February 2020 (English) |
| Iceland Icelandic Medicines Agency PFS symptoms referenced
|  | Mood swings such as depressive mood, depression and, rarely, suicide thoughts, have been reported in patients treated with STADA finasteride 1 mg... If you have any of these symptoms, stop taking STADA finasteride…and contact your doctor as soon as possible… Possible adverse reactions: permanent erectile dysfunction after stopping treatment; permanent decrease in libido after stopping treatment; permanent ejaculation problems after stopping treatment.—STADA finasteride package leaflet: December 2021 (English) |
| Ireland Health Products Regulatory Authority PFS symptoms referenced
|  | Reproductive system and breast disorders: Unknown: sexual dysfunction (erectile dysfunction and ejaculation disorders) that continued after discontinuation of treatment... Psychiatric disorders: Common: decreased libido. Unknown: depression, decreased libido that continued after discontinuation of treatment.—Finasteride 5 mg Summary of Product Characteristics: February 2014Mood alterations such as depressed mood, depression and, less frequently, suicidal thoughts have been reported in patients treated with finasteride 5 mg... If you experience any of these symptoms, contact your doctor for further medical advice as soon as possible.—Accord finasteride 5 mg package leaflet: November 2018 |
| Israel Pharmaceutical and Drug Information Department PFS symptoms referenced
|  | Mood alterations such as depressed mood, depression and, less frequently, suicidal thoughts, have been reported in patients treated with this medicine. If you experience any of these symptoms, stop taking ProAvenir and contact your doctor as soon as possible. Side effects of unknown frequency[:] persistent difficulty having an erection after discontinuation of treatment; persistent decrease in sex drive after discontinuation of treatment; persistent problems with ejaculation after discontinuation of treatment; male infertility and/or poor quality of semen; anxiety.—ProAvenir finasteride 1 mg Patient Information: March 2021 (Hebrew) |
| Italy Italian Drug Agency PFS symptoms referenced
|  | There have been case reports of patients experiencing: —Finasteride Safety Communication: November 2022 (English) |
| Japan Pharmaceuticals and Medical Devices Agency PFS symptoms referenced
| 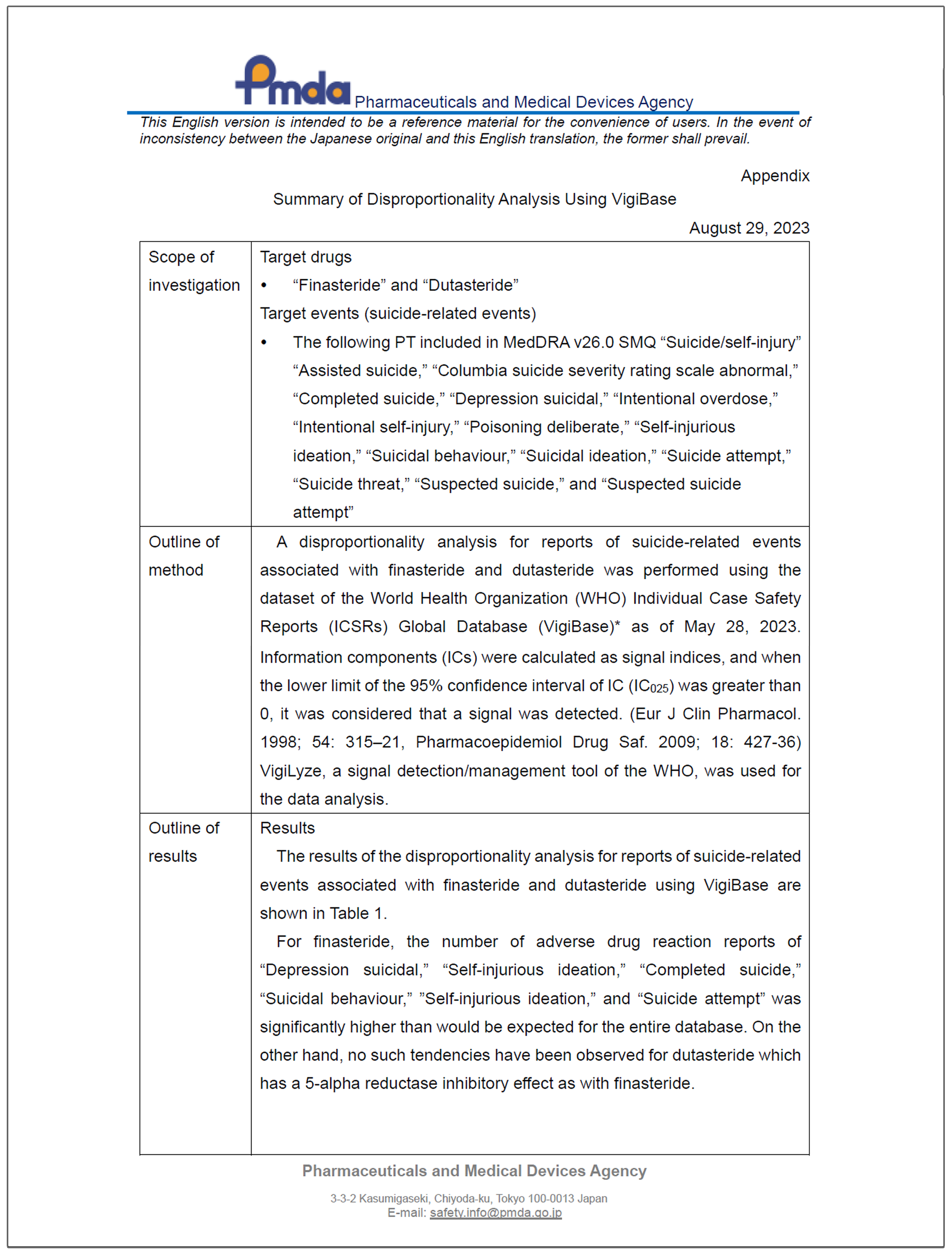 | A disproportionality analysis for reports of suicide-related events associated with finasteride… was performed using the dataset of the World Health Organization (WHO) Individual Case Safety Reports (ICSRs) Global Database (VigiBase) as of May 28, 2023. For finasteride, the number of adverse drug reaction reports of 'Depression suicidal,' 'Self-injurious ideation,' 'Completed suicide,' 'Suicidal behaviour,' 'Self-injurious ideation,' and 'Suicide attempt' was significantly higher than would be expected for the entire database. The results…suggested a relationship between finasteride and suicide-related events.—Summary of Disproportionality Analysis Using VigiBase: August 2023 |
| Jordan Food & Drug Administration PFS symptoms referenced
|  | [S]ide effects reported in some men [include] an inability to have an erection which may continue after stopping the medication, male infertility and/or poor quality of semen…depression, decrease in sex drive that may continue after stopping the medication, problems with ejaculation that may continue after stopping the medication, anxiety… Mood alterations such as depressed mood, depression and, less frequently, suicidal thoughts have been reported in patients treated with Proscar.—Proscar 5 mg package leaflet: May 2018 |
| Kazakhstan National Center for Expertise of Medicines and Medical Devices PFS symptoms referenced
|  | The [US Food and Drug Administration] has received information that after discontinuation of finasteride therapy, some patients have sexual side effects such as decreased libido, impaired ejaculation, decreased ejaculate, and impotence (in very rare cases)… According to a ruling by the FDA, the manufacturer included information on the decrease in libido and the possible development of male infertility while taking the drug.—Changes have been made to the finasteride patient instructions: March 2012 (English) |
| Latvia State Agency of Medicines PFS symptoms referenced
|  | [D]epression (feelings of severe sadness and unworthiness) including suicidal thoughts. These are uncommon side effects that have been reported with [finasteride]... If you have any of these symptoms, stop taking Accord finasteride 1 mg film-coated tablets and contact your doctor as soon as possible.—Accord finasteride 1 mg package leaflet: November 2021 (English) |
| Lithuania State Medicines Control Agency PFS symptoms referenced
| 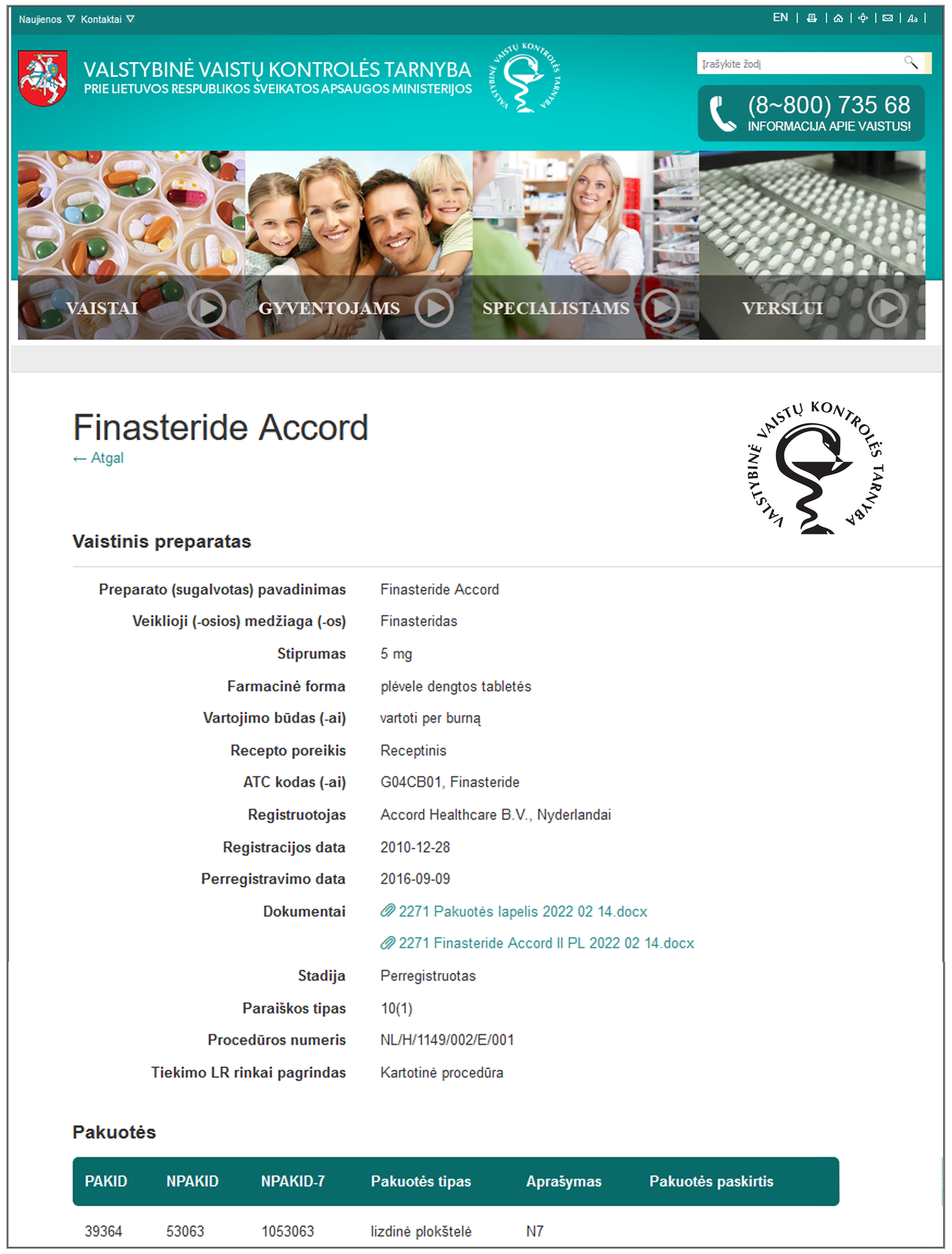 | Possible adverse reactions[:] permanent erectile dysfunction after stopping treatment, permanent decrease in libido after stopping treatment, permanent ejaculation problems after stopping treatment.—Accord finasteride 5 mg package leaflet: February 2022 (English)Psychiatric disorders: Depression, decreased libido which persists after stopping treatment, anxiety. Reproductive system and breast disorders: Testicular pain, erectile dysfunction that persists after stopping treatment; male infertility and/or poor sperm quality.—Accord finasteride 5 mg summary of product characteristics: February 2022 (English) |
| Malaysia National Pharmaceutical Regulatory Agency PFS symptoms referenced
| 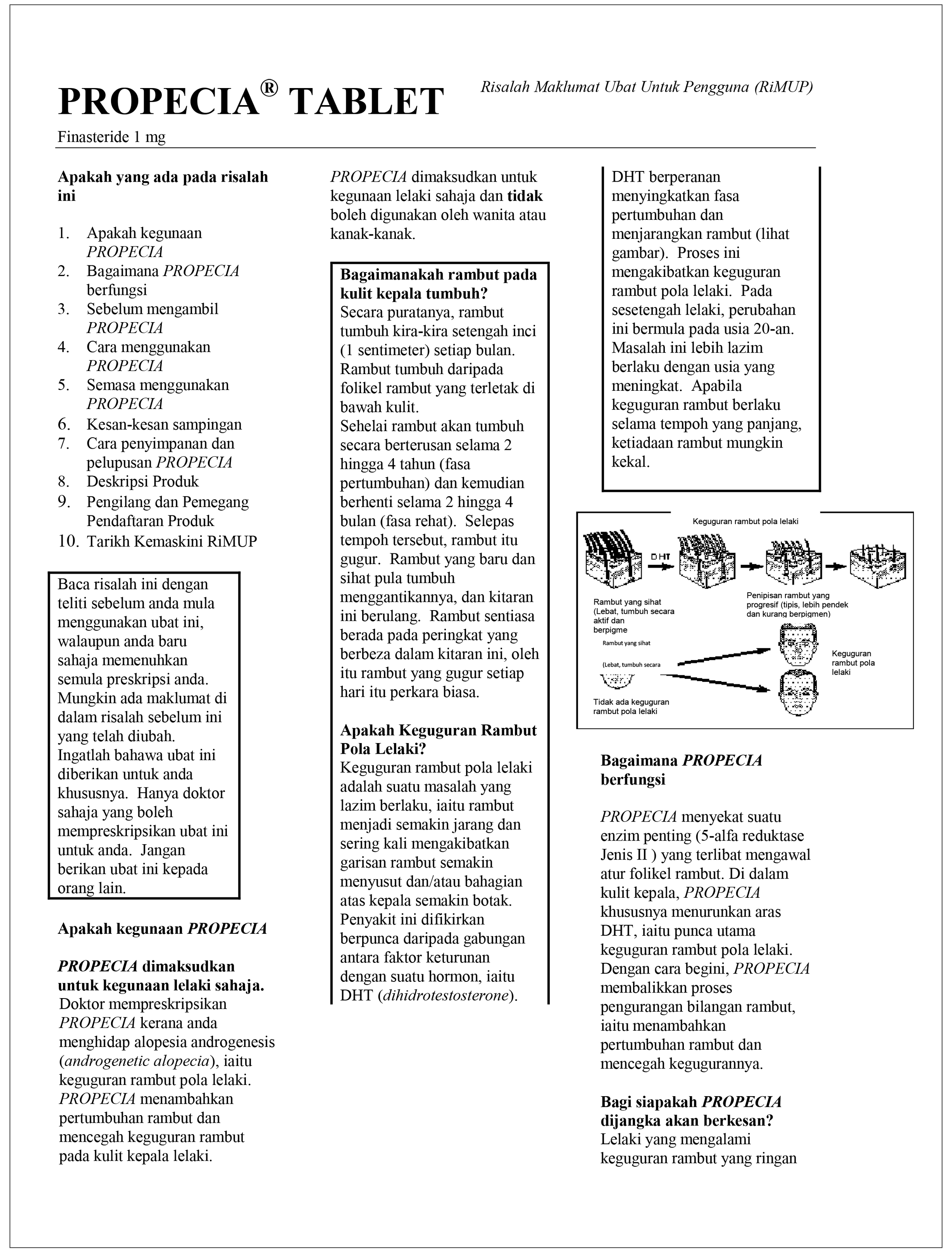 | Some men have also reported the following: allergic reactions such as rash; itching, hives and swelling of the lips, tongue, throat and face; testicular pain; an inability to have an erection that continued after stopping the medication; problems with ejaculation that continued after stopping the medication; male infertility and/or poor quality of semen. Improvement in the quality of semen has been reported after stopping the medication; depression; decrease in sex drive that continued after stopping the medication.—Proscar Consumer Medication Information Leaflet: January 2016 (English)In general use, the following have been reported infrequently: breast tenderness and enlargement; depression; decrease in sex drive that continued after stopping the medication; allergic reactions including rash, itching, hives, and swelling of the lips and face; problems with ejaculation that continued after stopping the medication; testicular pain; difficulty in achieving an erection that continued after stopping the medication; male infertility and/or poor quality of semen. Improvement in the quality of semen has been reported after stopping the medication.—Propecia Consumer Medication Information Leaflet: January 2016 (English)Additional source —Malaysian Adverse Drug Reaction Newsletter: August 2011 |
| Malta Medicines Authority PFS symptoms referenced
|  | [D]epressed mood, depression and, less commonly, suicidal thoughts have been reported in patients taking finasteride. Patients should be evaluated for psychiatric disorders and, if they occur, the patient should seek medical help… Reproductive system and breast disorders [include] sexual dysfunction that may persist after treatment is stopped.—Accord finasteride 1 mg package leaflet: April 2020Additional source —Accord finasteride 1 mg summary of product characteristics: February 2019 |
| Montenegro Agency for Medicines and Medical Devices PFS symptoms referenced
|  | Possible adverse reactions [include] permanent erectile dysfunction after stopping treatment, permanent decrease in libido after stopping treatment, permanent ejaculation problems after stopping treatment.—Proscar 5 mg patient instructions: August 2014 (English)Psychiatric disorders: Depression, decreased libido which persists after stopping treatment, anxiety. Reproductive system and breast disorders: Testicular pain, erectile dysfunction that persists after stopping treatment; male infertility and/or poor sperm quality.—Proscar 5 mg Summary of Product Characteristics: August 2014 (English) |
| Netherlands Medicines Evaluation Board PFS symptoms referenced
|  | Psychiatric disorders: Depression, decreased libido which persists after stopping treatment, anxiety. Reproductive system and breast disorders: Testicular pain, erectile dysfunction that persists after stopping treatment; male infertility and/or poor sperm quality.—Accord finasteride 1 mg Summary of Product Characteristics: February 2019 (English)Mood swings such as depressive mood, depression and, rarely, suicide thoughts, have been reported in patients treated with Accord finasteride... If you have any of these symptoms, stop taking Accord finasteride…and contact your doctor as soon as possible… Possible adverse reactions: permanent erectile dysfunction after stopping treatment; permanent decrease in libido after stopping treatment; permanent ejaculation problems after stopping treatment.—Accord finasteride 1 mg package leaflet: December 2021 (English) |
| New Zealand Medicines and Medical Devices Safety Authority PFS symptoms referenced
|  | Post-Finasteride Syndrome (PFS) was recently added to the US National Institutes of Health (NIH) list of genetic and rare diseases… PFS includes sexual, physical, and mental and neurological symptoms in patients who have taken finasteride. Symptoms often persist after the patient has stopped taking finasteride…Importantly, some patients can experience suicidal ideation and depression after stopping finasteride treatment. Patients and their families should be advised about these symptoms and to seek medical advice as soon as possible if they occur.—Medsafe Prescriber Update: April 2016Additional source —Propecia 1 mg package leaflet: August 2021 |
| Norway Norwegian Medicines Agency PFS symptoms referenced
|  | Psychiatric disorders: Depression, decreased libido which persists after stopping treatment, anxiety. Reproductive system and breast disorders: Testicular pain, erectile dysfunction that persists after stopping treatment; male infertility and/or poor sperm quality.—Actavis finasteride 5 mg Summary of Product Characteristics: September 2021 (English)Mood swings such as depressive mood, depression and, rarely, suicide thoughts, have been reported in patients treated with finasteride 1 mg film-coated tablets. If you have any of these symptoms, stop taking Accord finasteride…and contact your doctor as soon as possible… Possible adverse reactions: permanent erectile dysfunction after stopping treatment; permanent decrease in libido after stopping treatment; permanent ejaculation problems after stopping treatment.—Actavis finasteride 5 mg package leaflet: July 2020 (English) |
| Panama National Directorate of Pharmacy and Drugs PFS symptoms referenced
|  | Health Canada analyzed the risk of possible serious muscle-related side effects with the use of finasteride… At the time of the review, Health Canada had received 11 reports of serious muscle-related side effects suspected to be related to the use of finasteride… Health Canada’s review of the available information concluded that the risk of serious muscle-related side effects with the use of finasteride could not be ruled out.—Finasteride and the Potential Risk of Serious Muscle-Related Side Effects: August 2017 (English)Health Canada conducted a safety review for finasteride, due to reported cases of suicidal ideation and self-harm received in Canada and internationally. The cases led to the investigation of a possible relationship between finasteride use and suicidal ideation… Between 2012 and 2016, the reporting rate in Canada for finasteride-related adverse reactions and suicide/self-harm increased 2.5-fold.—Potential Risk of Suicidal Thoughts and/or Behaviors with Finasteride Use: May 2019 (English)Additional sources —Depression and Suicidal Ideation with Finasteride: November 2017 (English) —Adverse reactions associated with inappropriate use of finasteride: July 2014 (English) |
| Peru Directorate General of Drug Supplies and Drugs PFS symptoms referenced
|  | [T]he European Medicines Agency published the scientific conclusions referring to the evaluation of the Periodic Safety Reports for finasteride. The evaluation was carried out by the Pharmacovigilance Risk Assessment Committee (PRAC), which observed that during the reference period two serious cases were obtained for finasteride 5 mg, one in which suicidal behavior was reported and in the other case suicidal ideation. Cumulatively, 51 cases of suicidal ideation have been obtained according to the information in the summary table of adverse drug reactions during post-marketing use.—Director’s Resolution: March 2018 (English)After initiation of finasteride, observe patients for psychiatric problems. Advise patients to discontinue finasteride treatment if they develop mood disturbances, depression, and suicidal ideation… Instruct patients to inform their physician if they experience mood swings, depression and/or suicidal ideation while taking finasteride.—Risk of Mood Alterations, Depression and Suicidal Ideation: March 2018 (English) |
| Poland Office for Registration of Medicinal Products, Medical Devices and Biocidal Products PFS symptoms referenced
|  | Based on the reported cases of anxiety related to treatment with finasteride…and also taking into account that a high prevalence of anxiety in patients with MPHL has been confirmed, the PRAC considered that the adverse drug reaction anxiety should be included in…the Summary of Product Characteristics of all finasteride-containing products, with a frequency not known. The package leaflet is updated accordingly.—EMA Pharmacovigilance Risk Assessment Committee: February 2017 (English) |
| Portugal National Authority of Medicines and Health Products PFS symptoms referenced
|  | Interactions to keep in mind! Potentiation of depression, suicidal ideation with: finasteride, dutasteride.—INFARMED Pharmacovigilence Newsletter: September 2011Gynecomastia is not rare in adult men… [It] also appears as a side effect of medicines. The authors of this study undertook a systematic literature review encompassing articles from over one hundred publications. The result was the following list of medicines that are potentially associated with the occurrence of gynecomastia… finasteride.—INFARMED Pharmacovigilence Newsletter: November 2015Mood swings such as depressive mood, depression and, rarely, suicide thoughts, have been reported in patients treated with Accord finasteride... If you have any of these symptoms, stop taking Accord finasteride…and contact your doctor as soon as possible for additional medical help… Possible adverse reactions: permanent erectile dysfunction after stopping treatment; permanent decrease in libido after stopping treatment; permanent ejaculation problems after stopping treatment.—Accord finasteride 1 mg package leaflet: November 2016 (English) |
| Romania National Agency of Medicines and Medical Devices PFS symptoms referenced
|  | In patients treated with finasteride, mood changes such as depressed mood, depression and, less commonly reported, suicidal thoughts. If you notice any of these symptoms, consult your doctor immediately… Side effects can occur [including] inability to get an erection that persists after stopping treatment [and] decreased sexual drive that persists after stopping treatment.—Organon Proscar 5 mg package leaflet: October 2021 (English)Additional source —Organon Propecia 1 mg package leaflet: October 2021 (English) |
| Russia Federal Service for Surveillance in Healthcare PFS symptoms referenced
|  | [T]he Ministry of Health of the Russian Federation informs you that it is necessary to make changes to the instructions for use of…finasteride…according to updated information on experience based on their clinical application… The following information must be included… 'It is indicated that mood changes, including depression and suicidal ideation, were observed in patients taking finasteride at a dose of 5 mg. It is necessary to monitor the appearance of psychopathological symptoms and, when they take place, patients should be referred for consultation with a specialist.'—Finasteride label-change mandate: October 2017 (English) |
| Saudi Arabia Food and Drug Authority PFS symptoms referenced
|  | [T]he Saudi Food & Drug Authority inform[s] you about the below safety aspects[:] Patients should be aware of the risk of experiencing the adverse event (AE) of sexual dysfunction (including erectile dysfunction, ejaculation disorder, and decreased libido) during finasteride therapy. Patients should also be informed that case reports have been received of sexual dysfunction AEs that persisted after discontinuation of therapy...Healthcare Professionals should carefully monitor patients during treatment with finasteride for psychiatric symptoms (including anxiety, depression and suicidal ideation).—Confidential Merck DHPC finasteride warning letter: October 2018The weighted cumulative evidence identified from the reported cases, literature and data mining are sufficient to support a causal association between Finasteride and the risk of Diabetes Mellitus. Health regulators and health care professionals must be aware of this potential risk and it is advisable to monitor any signs or symptoms in treated patients.—Safety Signal of Finasteride and the Risk of Diabetes Mellitus: August 2021 |
| Serbia Medicines and Medical Devices Agency of Serbia PFS symptoms referenced
|  | In patients treated with finasteride [symptoms included] mood changes such as depressed mood, depression and, less commonly reported, suicidal thoughts. If you notice any of these symptoms, consult your doctor immediately… Side effects can occur [including] inability to get an erection that persists after stopping treatment [and] decreased sexual drive that persists after stopping treatment.—PharmaS finasteride 5 mg package leaflet: October 2021 (English) |
| Singapore Health Sciences Authority PFS symptoms referenced
|  | Mood alterations including depression and, less frequently, suicidal ideation have been reported in patients treated with finasteride. Healthcare professionals are advised to consider the potential risk of psychological AEs when assessing the benefit-risk of finasteride for their patients.—Finasteride and Potential Risk of Suicidal Ideation: September 2022 |
| Slovakia State Institute for Drug Control PFS symptoms referenced
|  | Reproductive system and breast disorders: sexual dysfunction (erectile dysfunction and ejaculation —Accord finasteride 5 mg Summary of Product Characteristics: January 2019 (English) |
| Slovenia Agency for Medicinal Products and Medical Devices PFS symptoms referenced
|  | Stop using [finasteride] and immediately contact a doctor if you experience any of the following symptoms[:] persistent difficulty having an erection after discontinuation of treatment, persistent decrease in sex drive after discontinuation of treatment, persistent problems with ejaculation after discontinuation of treatment.—Swedish Medical Products Agency: Finasteride PSUR: November 2013[T]he following have been reported in post-marketing use: persistence of sexual dysfunction (decreased libido, erectile dysfunction and ejaculation disorders) after discontinuation of treatment with finasteride.—Finasteride 1 mg Core Safety Profile: 2017Additional source —Finpros finasteride 5 mg package leaflet: July 2019 (English) |
| South Africa Health Products Regulatory Authority PFS symptoms referenced
|  | The following additional adverse experiences have been reported in post-marketing use[:] Depression, decreased libido that continued after discontinuation of treatment… Breast tenderness and enlargement, testicular pain, haematospermia, sexual dysfunction (erectile dysfunction and ejaculation disorders) that continued after discontinuation of treatment, male infertility and/or poor seminal quality.—Proscar 5 mg package leaflet: October 2018 |
| South Korea Ministry of Food and Drug Safety PFS symptoms referenced
|  | Warning: Mood swings including depressed mood, depression and suicidal thoughts were reported by patients treated with 1 mg of finasteride. Please make sure to observe patients for psychological symptoms. If any of the above symptoms are found in a patient, stop the treatment with finasteride and consult a medical provider.—Finasteride label-change mandate: July 2017 (English)Warning labels will be added to finasteride…noting that [it] may cause side effects including depression and suicidal thoughts, among other things. [T]he Ministry of Food and Drug Safety announced the warning…based on a safety report submitted by Merck Sharp & Dohme Korea, which originally developed the drug…Propecia was released in the Korean market in 2000. When its patent expired in 2008, several generic drugs were also released...There are presently more than 60 generic hair-loss drugs and 70 generic prostate-enlargement drugs on the market that have the same ingredients as Propecia.—Warning Against Depression and Suicidal Thoughts (Yonhap News): July 2017 (English)Additional sources —Korea FDA Drug Safety Information June 2012 (English) —Korea Institute of Drug Safety: Finasteride ADRs 2013-2016 (English) |
| Spain Spanish Agency for Medicines and Health Products PFS symptoms referenced
|  | Cases of mood disturbances, including depressed mood, depression and, less frequently, suicidal ideation have been reported in patients treated with finasteride 5 mg. This information will be included in the warning section of the drug’s data sheet together with the recommendation to monitor patients for the appearance of psychiatric symptoms and, if these occur, instruct them to seek medical advice.—Monthly Bulletin on Human Medicines: May 2017 (English)Psychiatric disorders: Depression, decreased libido which persists after stopping treatment, anxiety. Reproductive system and breast disorders: Testicular pain, erectile dysfunction that persists after stopping treatment; male infertility and/or poor sperm quality.—Normon finasteride 1 mg Summary of Product Characteristics: February 2018 (English)Additional sources —Organon Propecia 1 mg package leaflet: September 2020 (English) —Cinfa finasteride 5 mg: June 2021 (English) |
| Sweden Medical Products Agency PFS symptoms referenced
|  | In the SmPC [Summary of Product Characteristics] for Propecia, it is stated that adverse reactions usually have resolved themselves while on treatment or after cessation of therapy... This was supported by clinical data presented before the approval. After the approval, reported spontaneous reports received from [healthcare personnel] have regularly been presented... The most frequently reported adverse reactions...were erectile dysfunction, gynaecomastia and testicular pain. Within the cumulative submitted documentation, no new data has been presented indicating an increasing problem with male reproductive system symptoms...not being reversible after cessation of therapy.—Medical Products Agency (MPA) letter to Merck Sharp & Dohme (MSD): November 2006With regards to the MPA request to update section 4.4 of the SPC with information on the persistence of erectile dysfunction after cessation of treatment, MSD wishes to confirm our position that the pre-clinical, clinical and post-marketing data do not support inclusion of precautionary text in the SPC.—Merck response to MPA: June 2007The second report…concerned a 19-year-old male, who was placed on finasteride 1 mg... Subsequently the patient experienced erectile dysfunction, gynaecomastia, fatigue and feeling ‘mentally unclear.’ Finasteride was discontinued. The patient’s experiences persisted for almost two years [and] laboratory test results were normal.—MPA response to Merck: November 2007Comments on [labeling]: Section 4.8 should be revised according to the Guideline on Summary Product characteristics. The first sentence ‘Undesirable effects have usually been transient during treatment or resolved upon discontinuation’ should be deleted.—MPA Prel Renewal Assessment Report: April 2008Additional sources —Does Propecia Impair Sexual Function: December 2006 (English) —EMA Pharmacovigilance Risk Assessment Committee meeting: May 2019 —Accord finasteride 1 mg package leaflet: October 2019 (English) |
| Switzerland Swissmedic PFS symptoms referenced
|  | Reproductive system and breast disorders: sexual dysfunction (erectile dysfunction and ejaculation disorders) that persists after discontinuation of treatment... Psychiatric disorders: Common: decreased libido. Unknown: depression, decreased libido that continued after discontinuation of treatment.—Sandoz finasteride 5 mg Summary of Product Characteristics: December 2019 (English)Additional source —Sandoz finasteride 5 mg package leaflet: December 2019 (English) |
| Ukraine Department of Pharmacovigilance PFS symptoms referenced
|  | Reproductive system and breast disorders: sexual dysfunction (erectile dysfunction and ejaculation —Proscar 5 mg Summary of Product Characteristics: February 2019 (English) |
| United Arab Emirates Ministry of Health & Prevention PFS symptoms referenced
|  | Health Canada recently recommended adding a warning to products containing [finasteride]... While being administered the above-mentioned drug, patients may develop muscle weakness and stiffness, which in turn may cause pain to the patient, damage to muscle tissue and an increase in kinase enzymes. Accordingly, Health Canada and the UAE Ministry of Health recommend that companies that produce products containing [finasteride] add the above-mentioned warning to the internal leaflet of their products.—Adding a warning to pharmaceutical preparations containing finasteride: July 2017 (English) |
| United Kingdom Medicines and Healthcare products Regulatory Agency PFS symptoms referenced
|  | The MHRA completed a review of the safety of finasteride following concerns raised by patients regarding a lack of awareness of these side effects amongst patients and healthcare professionals. The MHRA reviewed the available evidence, including Yellow Card reports, published scientific literature and actions by other regulators [and] noted that the product information for finasteride contains information regarding the risk of depression and suicidal ideation and the potential of persistent sexual side effects after discontinuation with finasteride. However, these side effects do not appear to be well known by prescribers and patients, and therefore recommended the introduction of a patient card to increase awareness about these risks.—Men on Finasteride Asked to Stay Vigilant for Possible Psychiatric and Sexual Side Effects: April 29, 2024Some men have reported episodes of depressive illness in association with the use of Propecia... Some men also reported having suicidal thoughts. Depression and suicidal thoughts have been reported in men with and without a previous history of depression... A recent review of the evidence has suggested more significant depression can occur and so the advice is being updated to reflect this.—Finasteride: Rare Reports of Depression and Suicidal Thoughts: May 2017.Additional sources —MHRA Finasteride Public Assessment Report: July 2009 —Propecia 1 mg package leaflet: November 2017 —Dr. Reddy's finasteride 5 mg Summary of Product Characteristics: September 2018 —Drug Alerts: January 2020 —Promotion of finasteride by Careforsons Limited t/a Sons: December 2021 —Watchdog Launches Investigation into Hair Loss Pill as Men Report Huge Rise in Side Effects Including Depression, Low Libido and Erectile Dysfunction: Daily Mail, March 2023 —MHRA Finasteride Public Assessment Report: April 2024 |
| United States Food and Drug Administration PFS symptoms referenced
|  | What are the possible side effects of Propecia?Like all prescription products, Propecia may cause side effects. In clinical studies, side effects from Propecia were uncommon and did not affect most men. A small number of men experienced certain sexual side effects. These men reported one or more of the following:• less desire for sex; • difficulty in achieving an erection; and • a decrease in the amount of semen. Each of these side effects occurred in less than 2% of men. These side effects went away in men who stopped taking Propecia. They also disappeared in most men who continued taking Propecia. —Merck Propecia 1 mg Patient Information: August 2001 What are the possible side effects of Propecia?The most common side effects of Propecia include:• decrease in sex drive • trouble getting or keeping an erection • a decrease in the amount of semen The following have been reported in general use with Propecia: • breast tenderness and enlargement... • depression; • decrease in sex drive that continued after stopping the medication; • allergic reactions including rash, itching, hives and swelling of the lips and face; • problems with ejaculation that continued after stopping medication; • testicular pain; • difficulty in achieving an erection that continued after stopping the medication; • male infertility and/or poor quality of semen; • in rare cases, male breast cancer. —Merck Propecia 1 mg Patient Information: April 2012 What are the possible side effects of Propecia?• decrease in your blood Prostate Specific Antigen (PSA) levels...• There may be an increased risk of a more serious form of prostate cancer in men taking finasteride at 5 times the dose of Propecia. The most common side effects of Propecia include: • decrease in sex drive • trouble getting or keeping an erection • a decrease in the amount of semen The following have been reported in general use with Propecia: • breast tenderness and enlargement... • depression; • decrease in sex drive that continued after stopping the medication; • allergic reactions including rash, itching, hives and swelling of the lips, tongue, throat, and face; • problems with ejaculation that continued after stopping medication; • testicular pain; • blood in semen; • difficulty in achieving an erection that continued after stopping the medication; • male infertility and/or poor quality of semen. • in rare cases, male breast cancer. —Organon Propecia 1 mg Patient Information: June 2021 Postmarketing ExperienceThe following adverse reactions have been identified during post approval use of PROPECIA. Because these reactions are reported voluntarily from a population of uncertain size, it is not always possible to reliably estimate their frequency or establish a causal relationship to drug exposure:• Hypersensitivity Reaction: hypersensitivity reactions such as rash, pruritus, urticaria, and angioedema (including swelling of the lips, tongue, throat, and face); • Reproductive System: sexual dysfunction that continued after discontinuation of treatment, including erectile dysfunction, libido disorders, ejaculation disorders, and orgasm disorders; male infertility and/or poor seminal quality (normalization or improvement of seminal quality has been reported after discontinuation of finasteride); testicular pain; hematospermia. • Neoplasms: male breast cancer; • Breast disorders: breast tenderness and enlargement; • Nervous System/Psychiatric: depression, suicidal ideation and behavior. —Organon Propecia 1 mg Prescribing Information: August 2022 FDA is concerned that use of finasteride by pediatric patients may pose long-term safety risks regarding growth, development and sexual function. Any proposed use in the pediatric population would, of course, need to account for these safety concerns. Accordingly, your petition is denied.—FDA response to Merck’s Citizen Petition to approve finasteride for pediatric use: May 2000Additional source —Merck application for addition of depression to finasteride label: March 2011 |
